Neurobiologists at the University of Pittsburgh Brain Institute were awarded a four-year, $2.6 million grant from the National Institute of Neurological Disorders and Stroke to examine the connection between breakdowns in the blood-brain barrier and protein clumping in Alzheimer’s disease and related dementias.

Dr. Afonso C. Silva
The Pitt team will use the award to investigate, in mouse models of dementia, the effects of protein aggregation around blood vessels, explained principal investigator Dr. Afonso C. Silva, professor of neurobiology at Pitt School of Medicine. The blood-brain barrier is the brain’s “security fence,” as he puts it. It comprises tightly fitted cells that line the cerebral blood vessels and work with other kinds of specialized cells to protect the brain from harmful agents, while helping to maintain the brain’s unique environment.
“Blood-brain barrier dysfunction is common to many degenerative neurological diseases, including Alzheimer’s disease,” Silva said. “We don’t yet know whether this dysfunction occurs before the onset of neurological disorders or if it is a consequence of the diseases.”
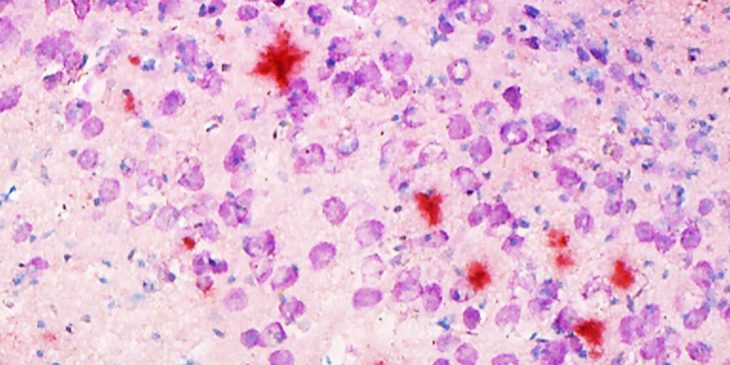
Other studies have indicated that blood-brain barrier dysfunction correlates with an abnormal deposition and aggregation of proteins, such as amyloid-b in Alzheimer’s disease and other proteins in a condition called Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy, or CADASIL. These proteins interact with the proteins that make up the extracellular matrix (ECM) and the basement membranes of cerebral blood vessels. The accumulation of protein deposits is thought to cause functional impairment of the ECM, fostering barrier breakdown and affecting regular transport of proteins. The problem gets worse over time, leading to cognitive impairment and death.
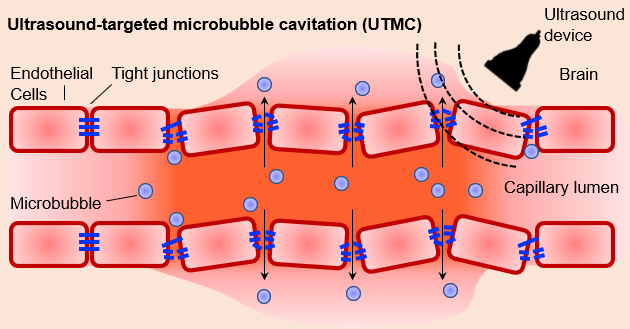
Researchers will use non-invasive ultrasound techniques to determine the impact of chronic disruption on the brain proteins. Credit: Dr. Afonso C. Silva
To show the impact of protein aggregation, the research team will detail the structural properties of the barrier in mouse models of Alzheimer’s and CADASIL by charting the degeneration and loss of various cell types and measuring the response of a variety of cellular proteins. They will track the functional properties of the blood-brain barrier as the mice age using MRI scans. They will use behavioral tests to quantify cognitive impairment and measure amyloid-b and other aberrant proteins in plasma, brain tissue and cerebrospinal fluid.
The researchers will also employ non-invasive ultrasound techniques to periodically create small defects in the barrier to determine whether chronic disruption affects the rates of protein deposition and aggregation in both healthy and disease-affected mice.
“Our experiments will provide crucial knowledge on the molecular and cellular mechanisms of interaction between protein aggregation and barrier breakdown, and may ultimately unravel novel therapeutic targets aimed at preserving brain health,” Silva said.