In early-stage breast cancer, overtreatment is a big problem. Because it’s unclear whose tumor will stay confined to the breast and whose will spread, the tendency is to treat everyone with some combination of chemotherapy, radiation and surgery.
To find better prognostic factors of breast cancer progression, Dr. Shilpa Sant, assistant professor of pharmaceutical science and bioengineering at the University of Pittsburgh, was awarded an NIH MERIT award totaling $2.2 million for the first five years with an option to extend two more years. Knowing the factors that promote tumor invasion and metastasis has value not only in diagnostics, but also in the realm of developing new treatments.
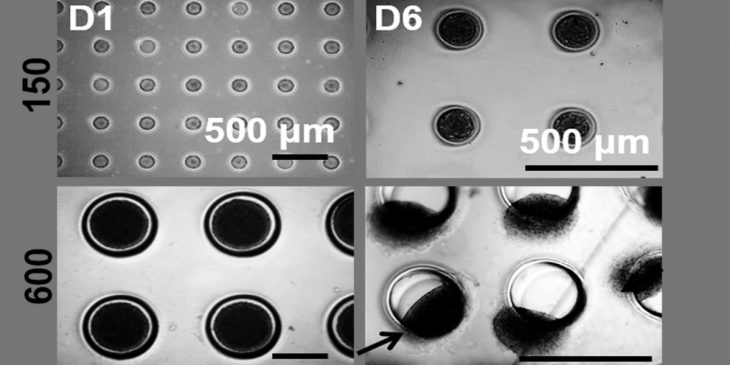
Most of the research in this area involves direct manipulation of the molecules known to influence tumor migration, either in 2D cell culture – which is structurally different from how tumors grow in the body – or live animal models. Though still using cultured cells, Sant takes a more natural approach, growing tumors in 3D microenvironments and monitoring them as they progress on their own. The tangible advantage of this approach is the ability to use patient-derived samples and to reduce the use of in vivo animal models.
“What we’ve observed is just by changing size of these tumors, they start migrating, even though they are composed of non-migrating, non-invasive parent cells,” Sant said. “As these in vitro tumors grow bigger, they send projections and start crawling out of their wells. We can see it in real time.”
The hypothesis is that nutrients and oxygen don’t make it into the core of a large tumor. These starving inner cells are fighting for survival, so they secrete signals to call for help from surrounding neighbors and the environment, which results in the emergence of migratory cells.
Then, to show that these signaling molecules are not just indicative of metastasis, but causal of it, Sant and her team used these molecules to transform the small, non-migrating tumors into migratory masses. They have also repeated these experiments in primary patient derived breast cancer cells.
The next step is to discover the factors secreted by tumor cells that promote tumor invasion and metastasis and look for similar factors in samples from breast cancer patients. Ultimately, the goal is to develop therapies, such as antibodies, that target those signaling pathways to stop cancer migration in its tracks.
Sant’s research team includes investigators from across the University of Pittsburgh, including Dr. Jianhua Xing, of the Department of Computational and Systems Biology; Dr. Vera Donnenberg, of the School of Medicine; Dr. Simon Watkins, of the Center for Biological Imaging; and Dr. Priscilla McAuliffe, of Magee-Womens Research Institute.