New research from the University of Pittsburgh sheds light on the development of complex immunological neighborhoods called tertiary lymphoid structures (TLS) in different tumor sites from patients with high-grade serous ovarian cancer (HGSOC), the most common and deadliest form of ovarian cancer.

Dr. Tullia Bruno
The formation of TLS, which orchestrate immune cell function for improved tumor control, is associated with better patient outcomes and response to immunotherapy. To better understand why these organized structures form less frequently in ovarian cancer compared to other solid tumors, Dr. Tullia Bruno, assistant professor of immunology at Pitt and UPMC Hillman Cancer Center, and Dr. Lan Coffman, associate professor of medicine at Pitt and UPMC Hillman, teamed up to compare the tumor microenvironment in tumors from the three most common sites of HGSOC growth — the ovary, fallopian tube and omentum, which is the layer of fatty tissue that lines the abdominal organs.

Dr. Lan Coffman
Bruno and Coffman elaborate on their findings, published recently in Cancer Cell, and explain how these insights could eventually lead to new treatment approaches for ovarian cancer.
What was the motivation for looking at TLS in ovarian cancer?
TB: TLS are understudied within multiple sites of disease in ovarian cancer patients. More importantly, only 10% to 15% of ovarian cancer patients form these complex immunological neighborhoods in tumors whereas in other solid tumors the formation is over 40%. Thus, we are interested in driving TLS formation in ovarian cancer patients for increased immune function within tumors.
What were the main findings of this study?
TB: We found that TLS formation is markedly different depending on the site of cancer. Specifically, fallopian tube and omental tumors have increased TLS formation and activity compared to ovarian tumors. Another important finding was that B cells, the white blood cells that make antibodies, from ovarian tumors didn’t multiply well and made less effective antibodies than B cells from fallopian tube and omental tumors.
Were you surprised by any of your findings? Why or why not?
LC: The striking differences in TLS frequency and features based on tissue location, particularly in the fallopian tube vs. ovary, was surprising. We don’t have a good understanding of how the underlying tissue, particularly in two anatomically similar gynecologic organs, impacts the immune response, so it was intriguing to see such a tissue-dependent difference.
TB: In addition to cancer and immune cells, the tumor microenvironment is made up of stroma, a diverse group of cells that influence tumor metabolism, growth, metastasis and treatment resistance. Stroma is like the soil in which seedlings (immune cells) and weeds (tumor cells) grow. Just as soils are composed of different minerals and organic matter that support different plant species, tumors have stromal cells that help or hinder immune and tumor cells.
We were struck by how stromal cell populations, particularly cancer-educated mesenchymal stem cells, increased with reduced TLS formation. These suppressive cells also reduced the ability of B cells to function and were blunted in their ability to become supportive stromal populations that are necessary for TLS formation.
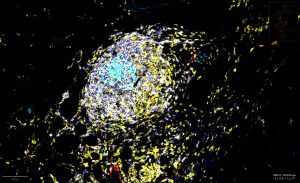
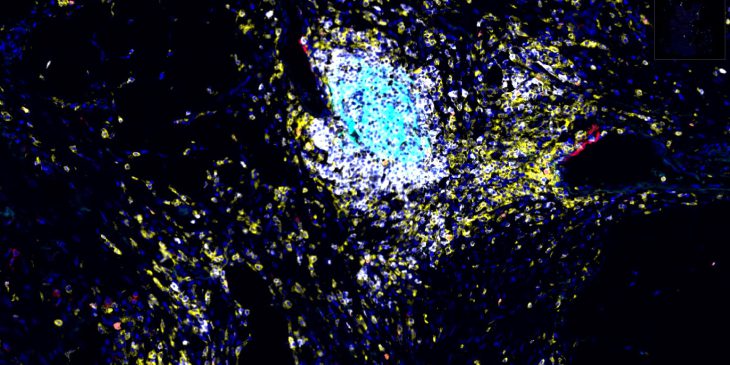
The activity of tertiary lymphoid structures in ovarian cancer is driven by site and stroma (CREDIT: Ian MacFawn, Bruno Lab)
Why are these findings important, and what are the clinical implications?
LC: Ovarian cancer is a disease with limited treatment options and poor outcomes overall. Thus far, immunotherapy has largely failed in ovarian cancer despite tremendous success in other solid cancers. By detailing a critical aspect of the ovarian cancer microenvironment, this work advances our understanding of how stromal and immune cells interact to modulate anti-cancer immunity. The overall goal is to translate these new findings into novel treatment which successfully harnesses the power of immunotherapy to improve the lives of women with this deadly disease.
What’s next for your research?
TB and LC: A clear next step is to create supportive stromal niches in ovarian cancer patients for improved TLS formation and function. This approach, which involves modulating non-immune cells to increase immune function within suppressive tumor microenvironments, is a fundamentally different way of thinking about immunotherapy. It’s like fertilizing the soil to nurture beneficial immune responses.
Is there anything else you’d like to mention?
TB: It has been amazing to work together on this complex question. We have combined the expertise of both of our research groups to tackle these important biological questions, which is a true testament to why team science is so important. An added bonus has been working with such an incredible woman in science.
Journalists interested in learning more can contact mediarelations@upmc.edu.