Imagine a battlefield medic rushing up to a battered soldier. Now picture an emergency medical technician racing toward an overturned car off a rural road. In both cases, the victims are severely bleeding.
The medic and the EMT face the same two problems: They are far from the closest trauma center, and neither are likely to be carrying cold bags of life-saving whole blood.

Graphic rendering of dried red blood cells in an IV bag
Now imagine they reach into their medical packs and pull out flat IV bags with a red powder inside. They add some water, and, within moments, they’re transfusing artificial whole blood into the patient, buying precious time for transport to a trauma center.
This week, a team of University of Pittsburgh scientists and UPMC surgeons announced that they are a key part of a national $46.4 million Defense Advanced Research Projects Agency (DARPA)-funded consortium that aims to create shelf-stable, artificial dried whole blood with the potential for use in humans within a decade, making desperately needed blood available where it is not today.
“Roughly 30,000 people die a preventable death from traumatic bleeding every year in the U.S.,” said Dr. Philip Spinella, professor of surgery and critical care medicine and associate medical director of the Center for Military Medicine Research at Pitt. “These patients are predominantly dying in the prehospital phase of resuscitation due to the lack of availability of blood products. Whole blood is the optimal strategy for massive bleeding. A dried artificial whole blood will give emergency medics the chance to save tens of thousands of lives per year because waiting for hospital treatment is often too late.”
Because liquid whole blood expires within weeks and must be kept cold, it would be wasteful, expensive and logistically challenging to stock every ambulance with it. Lightweight bags of “just add water” blood that don’t expire for years is the goal of the new project, called Fieldable Solutions for Hemorrhage with bio-Artificial Resuscitation Products (FSHARP) and managed through the University of Maryland School of Medicine.

Dried red blood cells
There are three main components of whole blood: red blood cells that carry oxygen, platelets that form clots and stop bleeding, and plasma that carries proteins, salts, sugars and fats.
The consortium aims to combine dried artificial red blood cells made by KaloCyte Inc. (co-founded by Spinella), artificial platelets made by Haima Therapeutics (Spinella and co-investigator at Pitt, Dr. Matthew Neal, serve on the scientific advisory board) and freeze-dried plasma that has been federally approved for use by the U.S. military.
Beyond aiding paramedics in saving traumatically injured people, which is the primary goal for the DARPA program, subsequent work may be devoted to customizing artificial dried whole blood to better treat patients depending on what is causing them to lose blood, whether it be postpartum bleeding after birth, a gastrointestinal bleed or a traumatic brain injury, said Neal, a UPMC trauma surgeon and Roberta G. Simmons Associate Professor of Surgery at Pitt’s School of Medicine.

From left to right: Drs. Philip Spinella, Matthew Neal, Susan Shea
“Trauma medicine is at least 30 years behind oncology when it comes to personalized medicine,” said Neal, who also co-directs Pitt’s Trauma and Transfusion Medicine Center with Spinella. “I want us to do for trauma patients what we’re doing for cancer patients.”
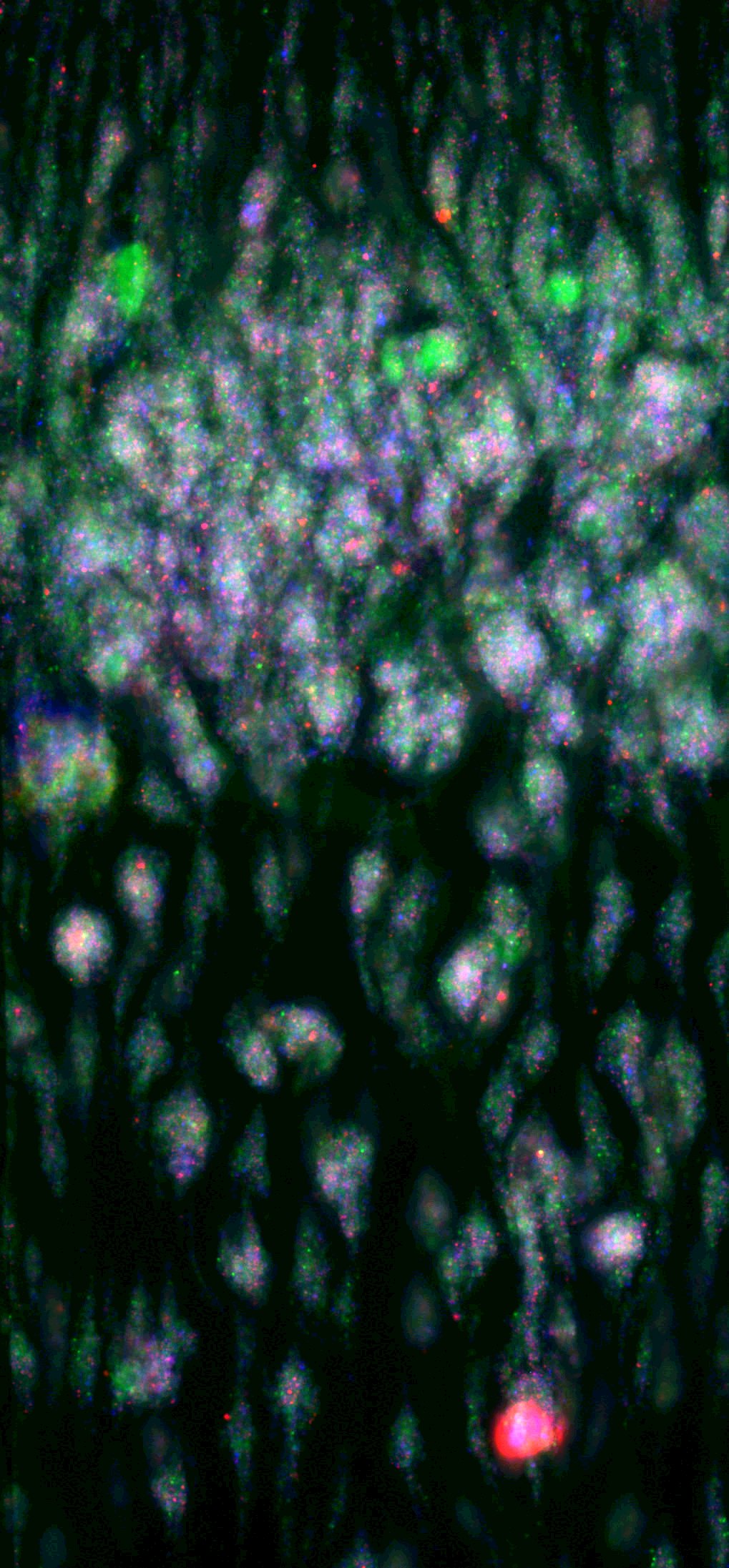
Blood clot in a microfluidic device taken in Dr. Susan Shea’s laboratory as part of her team’s research into how blood clots and flows.
For the initial phase of the FSHARP project, the Pitt scientists will be in charge of creating the blood bank that will be used by all research labs in the program to compare function between liquid whole blood and the dried artificial whole blood product. The team will also be performing laboratory-based tests using microfluidics, a tool to study blood behavior in micro-channels that mimic vessels in the human body. Dr. Susan Shea, assistant professor of surgery and bioengineering at Pitt, directs that part of the project.
“Before we can test the artificial blood in humans, we have to know everything about how it works, down to the most microscopic clot,” Shea explained. “If the artificial dried whole blood doesn’t clot just right, it can cause more harm than good. In my lab, we’re putting the artificial blood up against the most rigorous clinical tests that any physician treating a bleeding patient would use.”
Neal, exhausted after a night on service caring for patients, described an experience where the shelf-stable artificial blood would have been perfect.
“A patient was transferred in from a small hospital just a few hours away, massively bleeding from their injuries. That hospital had only two units of blood available when 10 were needed,” he said. “That is the problem we are going to fix.”