A paper published today in Molecular Cell lays out what happens when a key chromosome maintenance pathway is inhibited, providing a glimpse into the decisions cancer cells make when they are unable to grow indefinitely.
Cancer cells must activate a telomere elongation pathway to ensure that as they replicate over and over again, the ends of their chromosomes remain intact.
For about 15% of all cancers, the alternative lengthening of telomeres (ALT) pathway is the method of choice. That includes some of the cancers with the worst prognosis for patients.
“About half of glioblastoma, 40% of pancreatic, 40% of neuroblastoma – all of these nasty ones — activate the ALT pathway,” said senior author Dr. Roderick O’Sullivan, assistant professor of pharmacology and chemical biology at the University of Pittsburgh. “Basically, if you could inhibit ALT, you have a new way of attacking these cancers.”
But knowing the pathway isn’t enough. Researchers have spent decades searching for the smoking gun – the specific proteins that cancer cells are hijacking to gain immortality through telomere elongation.
This paper characterizes the first such protein.
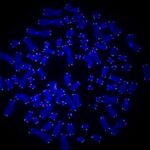
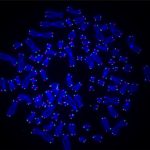
The researchers, based at UPMC Hillman Cancer Center, used CRISPR to delete the RAD51AP1 gene, which codes for a DNA repair protein of the same name that was identified in an earlier study.
RAD51AP1 creates a bridge between the telomeres so they can recombine, filling in any gaps left by the DNA replication process.
Without the RAD51AP1 protein, the telomeres started showing damage.
In normal healthy cells, telomere damage causes them to stop growing – a process called senescence. O’Sullivan’s team thought the same would happen in the gene-edited cancer cells, but it didn’t.
Instead, the cells activated a self-preservation mechanism known as autophagy, where the most unfit cancer cells eat themselves from within to recycle precious metabolites for other cells to survive.
When the researchers shut down autophagy as well, though, that’s when the cancer cells started to die.
In the future, combined therapy that blocks ALT telomere elongation and autophagy could be used to treat some of the most devastating cancers, many of which afflict children.