Tucked away in a small, dark room at UPMC Hillman Cancer Center, Brittani Schnable is on a fishing expedition.
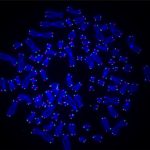
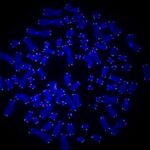
Wielding a joystick similar to those used by video gamers, she casts microscopic beads into an ocean of molecules, pushing and pulling the beads apart until they eventually catch a strand of DNA. After a few taps of the keyboard, a lightshow begins. A burst of colors flashes across the black screen like fireworks exploding in the night sky.
Although these colors seem random at first, a pattern starts to emerge. Lines of blue and red light streak across the screen: A DNA repair protein has bound to the site of damage.
Schnable, a Ph.D. student in Dr. Bennett Van Houten’s lab at the University of Pittsburgh, is using cutting-edge technology called a C-trap that manipulates a single molecule of DNA and a new method — described this week in Nucleic Acids Research — allowing for quick and easy production of proteins for single-molecule visualization.
The novel system gives Van Houten and his team an unparalleled level of detail that will help them explore how cells find and repair damaged DNA, information that could someday be used to halt cancer in its tracks.
The video shows a molecular lightshow: A strand of DNA with UV damage is strung between two beads on the C-trap. This sped-up video shows DNA repair proteins DDB1 (blue) and DDB2 (red) binding and unbinding to sites of damage separately or together (purple) over about 12 minutes (CREDIT: Schaich et al., 2023, Nucleic Acids Research, doi:10.1093/nar/gkad095)
“I like to think about DNA damage as a pothole,” said Van Houten, professor in Pitt’s Department of Pharmacology & Chemical Biology. “In one particular DNA repair pathway, it takes about 30 proteins to go from finding the pothole to putting in the repair patch. While we can’t observe all of these proteins at once, we can observe them two by two.”
Van Houten’s lab is interested in repair proteins that mend DNA lesions caused by environmental factors, such as ultraviolet (UV) radiation from the sun and environmental pollutants. If these repair pathways break down, DNA damage can contribute to aging, cancer, neurodegeneration and other diseases.
In the new study, the researchers used the C-trap to investigate how different DNA repair proteins identify and bind to their respective forms of damage.
The C-trap system draws on Nobel prize-winning technology called optical tweezers, which use a strong beam of light to grasp and move microscopic beads until they stick to either side of a molecule — in this case a strand of damaged DNA.
“You can move the two beads together and hope that the two DNA ends latch on to each bead like Velcro. When you move the beads further apart, you can actually feel the measure of force of DNA like a spring, or a rubber band,” said first author Dr. Matthew Schaich, a postdoctoral fellow in the Van Houten Lab.
Once the DNA bait is set, it’s time to go fishing for proteins.
In collaboration with University of Kent researchers, the Van Houten group developed a new method called Single Molecule Analysis of DNA-binding proteins from Nuclear Extracts, or SMADNE. This technique allows users to create fluorescently tagged proteins much faster and with greater ease than traditional methods. Using SMADNE, the researchers extracted DNA repair proteins from the nucleus of the cell. They then introduced these proteins into the C-trap and analyzed how and when they bound to DNA containing various types of damage.
Dr. Bennett Van Houten, Brittani Schnable and Dr. Matt Schaich (from left to right) with the C-trap instrument at UPMC Hillman Cancer Center
Interested in the relationship between two particular repair proteins, DDB1 and DDB2, which help repair damage caused by the sun, Schaich watched these proteins popping on and off the DNA as flecks of multicolored light and studied the way they approached and retreated from the site of UV damage.
“You have an area of DNA damage, and you want to know how a cell can identify and fix it,” Schaich explained. “One of the most important things to understand is who gets there first. Once it arrives, does it stay around for the whole repair cascade? Does it hand off repair to a different protein? With the C-trap, you can watch the proteins coming and going and learn a lot about the orders of assembly and disassembly.”
Van Houten thinks about DNA repair proteins like people socializing at a bar.
“Two people walk into a bar. Who goes through the door first? How long do they sit together at the bar, and then who leaves the bar first? DNA repair proteins, like people, are dynamic,” said Van Houten.
The researchers found that when DDB1 and DDB2 were working together at the damage site, they usually arrived at the DNA together and departed together, as expected. Yet, surprisingly, they also saw 11 different association and dissociation patterns with the two proteins arriving and leaving at different times, highlighting the incredible detail that scientists can observe using this new technology.
In addition to DDB1 and DDB2, Van Houten’s team used the C-trap and SMADNE to investigate the activities of a plethora of DNA repair proteins from several different repair pathways in an effort to improve understanding of these repair systems.
By learning how our DNA repair processes work, scientists can better understand how disruptions in these pathways can lead to diseases such as cancer and advance the search for better treatments.
“DNA repair is a double-edged sword,” Van Houten explained. “If you don’t have efficient repair, environmental stressors could cause enough damage that cancer develops. On the other hand, many cancer treatments kill tumors by targeting DNA repair mechanisms.”
Van Houten and his team have applied for a patent for their SMADNE system and will continue to analyze all 30 of the proteins in this UV damage repair pathway.
“The combination of the C-trap and SMADNE has opened up endless opportunities for the study of DNA repair. But what’s the most important question that we can answer using this new tool?” said Van Houten. “To me, it’s knowing the precise role of each of the proteins in this pathway.”
Hayley Rein is a Ph.D. Candidate and Pre-Doctoral Fellow in the University of Pittsburgh School of Medicine’s Molecular Pharmacology Program. She is participating in the UPMC Science Writing Mentorship Program.
For more information: mediarelations@upmc.edu