Hospitals are where people go to get better. But being hospitalized increases the risk of unwanted complications – including infections.
And when a vulnerable patient gets an infection, the consequences can be dire. That’s why Dr. Partha Biswas is taking a deep dive into the biological mechanisms our bodies use to resist infections, with the hope that someday that response can be boosted – giving patients a fighting chance against our microscopic foes.
“Arguably the most urgent medical challenge of our time is hospital-acquired infections and growing germ resistance to our dwindling arsenal of antimicrobials. An infection can kill a hospitalized patient in 48 hours – too fast for doctors to even determine what pathogen has infected them,” said Biswas, associate professor of rheumatology and clinical immunology at the University of Pittsburgh School of Medicine. “So, what if, instead of playing catch-up and treating an infection after it is causing damage, we can prepare the patient to fight off potential pathogens by boosting their immune system?”
Usually when a patient gets an infection, their doctor must send a sample to pathology where it is grown in a cell culture to determine what pathogen is causing the infection. Knowing this, the doctor can then prescribe a medication tailored to destroy that particular germ. But diagnosing the infection can take days – precious time when the infection can spread unchecked.
In the April 13 issue of Cell Host & Microbe, online today, Biswas explains how he and a team of immunologists, pharmacologists and infectious diseases experts uncovered a cellular pathway that may prompt a faster immune response and help avoid catastrophic infections, the third most common cause of death in hospitalized patients.

Dr. Dedong Li and Dr. Partha Biswas
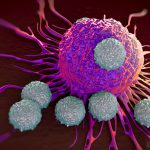
The key is to focus on neutrophils, the first immune cells to respond to infection. Neutrophils are capable of fighting a wide array of infectious agents, but because they do not target a specific pathogen, they can also cause damage to the patient’s tissue if left unchecked. This means that the activity of neutrophils must be tightly regulated.
Neutrophils release unstable molecules that create net-like structures that intoxicate and trap pathogens. But this process requires a tremendous amount of energy, which the neutrophils obtain by absorbing glucose – or blood sugar – from their surroundings in what’s known as a metabolic process.
Biswas theorized that if scientists have a detailed understanding of each step in the neutrophil metabolic process, they may be able to find a step that can be turned on and off as needed to fight a burgeoning infection before it gets out of control.
Through a series of experiments involving a type of yeast called Candida albicans, which is often involved in hospital-acquired infections, Dr. Dedong Li, a postdoctoral fellow in the Biswas lab, led the team in mapping out the cellular process for neutrophil activation. They found GLUT 1 – the protein responsible for transporting glucose to the neutrophils – to be an attractive target.
Mice whose neutrophils were unable to produce GLUT1 could not effectively produce the unstable molecules needed to “trap” C. albicans, the team found. They then showed that neutrophils in human kidney cells also require GLUT1 production similar to mouse neutrophils.
Biswas expects the research findings will lead to the development of drugs that could help activate or supplement neutrophils in patients whose immune systems are depleted by either their disease or treatment – such as chemotherapy or immunosuppressant medications. He envisions this occurring at either the first sign of infection or even as a preventive measure when patients who are particularly vulnerable to infection are first admitted to the hospital.
“The irony is, despite all our incredible advances in medicine, we still depend on traditional, old-school diagnostics that take at least 72 hours in order to find the right treatment for a patient with an infection,” Biswas said. “That’s far too long. Finding a solution starts with understanding the cellular process. Drug development and clinical trials will follow. It is heartening to use science to solve this mystery and set medicine on the path toward a life-saving solution.”
Laura Bahr is a graduate student researcher in the University of Pittsburgh School of Medicine’s Department of Cell Biology and Molecular Physiology. She is participating in the UPMC Science Writing Mentorship Program.