Rising rates of vaccinations against COVID-19 across Pennsylvania and the country are certainly cause for celebrating – and bring much-needed hope for the return of the kind of summer activities and gatherings that we’ve all longed for. But as our own data already show, some vaccinated people remain extremely vulnerable to the virus – and a still sizable number of Americans indicate they may not get vaccinated at all.
That’s why any “return to normal” will require multiple strategies for protecting the people in our communities. At UPMC, we’ve particularly focused on providing a highly effective treatment for COVID-19 called monoclonal antibodies.
These are human-made substances that act like natural antibodies, the proteins your immune system makes to help defend against viruses and bacteria. Human-made antibodies have previously been used to treat conditions that include certain cancers and some autoimmune diseases, like rheumatoid arthritis. In 2020, several monoclonal antibodies were developed by pharmaceutical companies to target SARS-CoV-2, the virus that causes COVID-19.
These antibodies attach to the spike protein on the virus and block it from entering the cells. Based on early data that showed them to be a promising treatment for mild to moderate COVID-19, the Food and Drug Administration last year authorized monoclonal antibodies for use in patients with the disease.
There are currently two different combinations of drugs that are available at multiple UPMC sites across the communities we serve. At this time, both are believed to be equally effective against the virus, including the variants you may have heard about in the news, but we are closely studying any differences in outcomes.
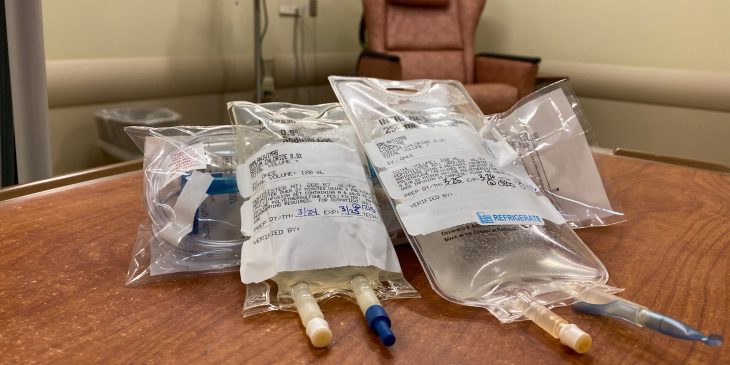
Monoclonal antibody treatments are given through an intravenous infusion as a one-time dose over about 30 minutes in one of our infusion centers or emergency departments. This is followed by a period of observation for side effects, which are typically mild and include nausea, diarrhea and headache. More serious reactions are very rare.
UPMC has treated over 1,300 patients with this transformative therapy. In our experience, monoclonal antibodies have dramatically improved outcomes for a large number of patients. They have greatly decreased the rate of hospitalization and death.
One couple that sticks with me are Julie and Irv Freeman. They’d both been very careful, but Julie, age 64, tested positive for COVID-19 in mid-January and became quite ill. They knew about monoclonal antibodies and knew she qualified. Fortunately, they connected with me and our fantastic team was able to schedule Julie for treatment in less than 24 hours. She made a full recovery within days. When her 64-year-old husband tested positive a week later with mild symptoms, he didn’t hesitate to get scheduled for monoclonal antibodies. His symptoms never progressed beyond a little congestion and fatigue.
The Freemans are dedicated to spreading the word about their experience with monoclonal antibodies and encouraging others to consider it if they get COVID-19.
Many people who test positive for COVID-19 are eligible for this treatment but don’t even know it. If you have been diagnosed with diabetes, kidney disease, are overweight or have decreased function of your immune system, you may be eligible. Also, if you are between the ages of 12 and 17 with certain medical conditions, or older than 55 with heart disease, high blood pressure or lung disease, you may be eligible. And simply being age 65 or older qualifies you for the treatment if it’s offered within 10 days of the onset of symptoms.
You are eligible for this treatment – offered at no cost by UPMC Health Plan and many other insurers – even if you have been previously vaccinated. If you do receive monoclonal antibody treatment, you should not receive any additional vaccinations for COVID-19 within 90 days of the treatment.
To learn more, visit UPMC.com.
Dr. Wadas is executive vice chair of community emergency medicine at UPMC.