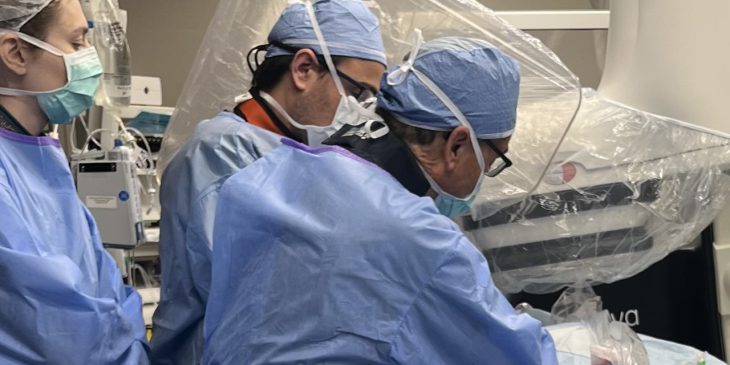
A team of physicians at the UPMC Heart and Vascular Institute (HVI) recently became the first in Pennsylvania to implant a new generation of miniaturized pacemakers that reduce complications and improve convenience for patients.
The leadless Medtronic devices, which received Food and Drug Administration approval in May, provide longer battery life and more sophisticated programming than previous devices, noted Dr. Sandeep K. Jain, director of cardiac electrophysiology at UPMC HVI.
Because of the longer battery life of these pacemakers, the world’s smallest, patients who receive one are projected to need only one device for life. And the advanced programming means fewer in-office visits to make adjustments. It’s estimated that up to 3 million people in the United States have pacemakers, and that number is growing rapidly in an aging population.
“At UPMC, we continuously strive to introduce innovative technologies that will bring meaningful benefits to our patients,” said Dr. Alaa Shalaby, who implanted the device in the first patient in May. “Our highly skilled team at HVI is excited to lead the way in the state by offering this new capability to the communities we serve.”
Comparable in size to a multivitamin, Medtronic’s Micra pacemakers are less than one-tenth the size of traditional pacemakers. Unlike traditional models, they do not require leads — wires to the heart — or a surgical “pocket” under the skin, so potential sources of complications are eliminated, and there is no visible sign of the device. Pacemakers send mild electrical signals to the heart to keep it beating normally and to help stop the dizziness, fainting and shortness of breath caused by a slow heart rate.
“This latest milestone underscores our mission at HVI,” said Jain. “Our multidisciplinary team of experts uses the latest technology, conducts cutting-edge research and participates in the latest clinical trials to give our patients access to treatments that may not be available at other hospitals.”