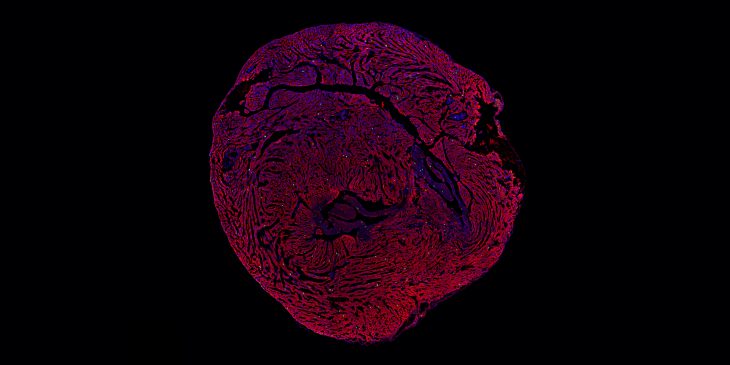
Heart failure can occur later in life because the adult heart cannot repair itself after multiple injuries.
A new study from the laboratory of Bernhard Kühn, M.D., associate professor of pediatrics at the University of Pittsburgh and director of cardiology research at UPMC Children’s Hospital of Pittsburgh, reveals why heart muscle cells, also known as cardiomyocytes, are limited in their ability to regenerate.
The study, published recently in Developmental Cell, shows that the limiting factor is a protein called Lamin B2, which resides on the outer layer of the cell’s nucleus. The researchers found that heart muscle cells stop dividing in adult mice because they lack enough of the Lamin B2 protein. In mice genetically engineered to express more Lamin B2, heart muscle cells began to replicate again, and heart tissue regenerated itself.
“When the heart has an injury like myocardial infarction, this can, in the long term, cause heart failure,” said lead author Lu Han, Ph.D., a research scientist in pediatrics at Pitt. “One of the major reasons is because the heart muscle cells cannot proliferate.”
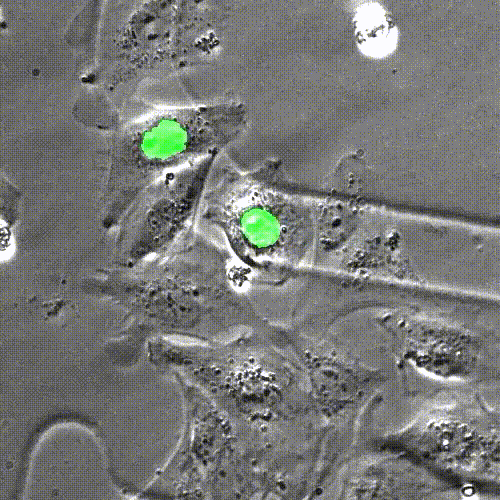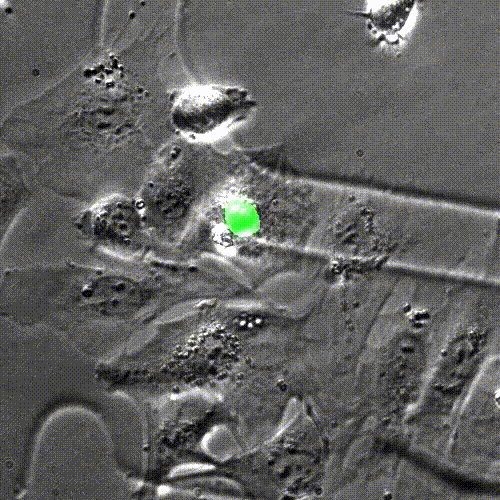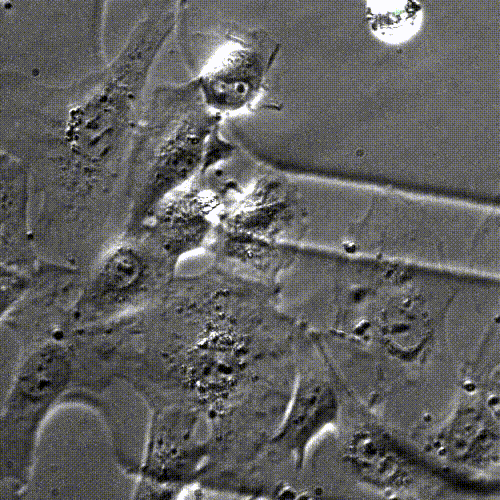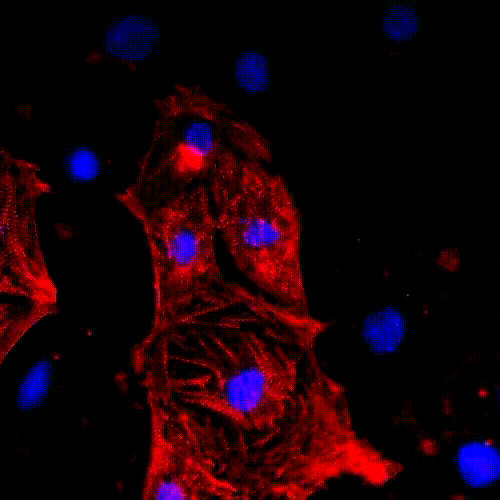
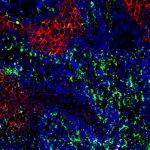
Cell division (mitosis). Credit: Lu Han, Ph.D.
Tissue regeneration requires a process called mitosis, where cells divide and proliferate. For many types of cells in the body, mitosis happens frequently. Skin cells, for example, are constantly dividing and proliferating to restore the skin’s outer layer. In contrast, most heart muscle cells stop proliferating after infancy.
This study showed how heart muscle cell division decreases because the cells lose Lamin B2, causing them to get stuck together when they try to divide, meaning they have more than two copies of the organism’s DNA-containing chromosomes in each nucleus. Once these extra chromosomes are present, cell division is not so simple anymore.
To figure out what was blocking cell division, the researchers analyzed the genes present in heart muscle cells in their proliferative versus non-proliferative states. They found that the Lamin B2 gene was highly expressed in newborn mice, whose heart cells were still proliferating, but not as much in adult mice. Using genetically engineered mice, they showed that the presence of Lamin B2 was necessary for the heart muscle cells to complete their cycles of cell division. Eliminating the Lamin B2 gene prevented the cells from completing the cell division cycle. This caused accumulation of extra copies of the cell’s DNA.
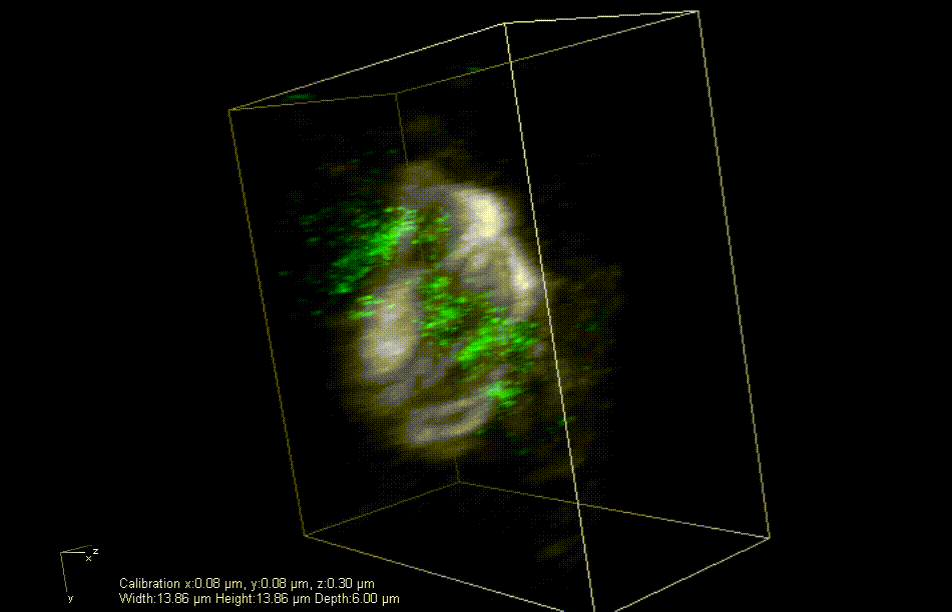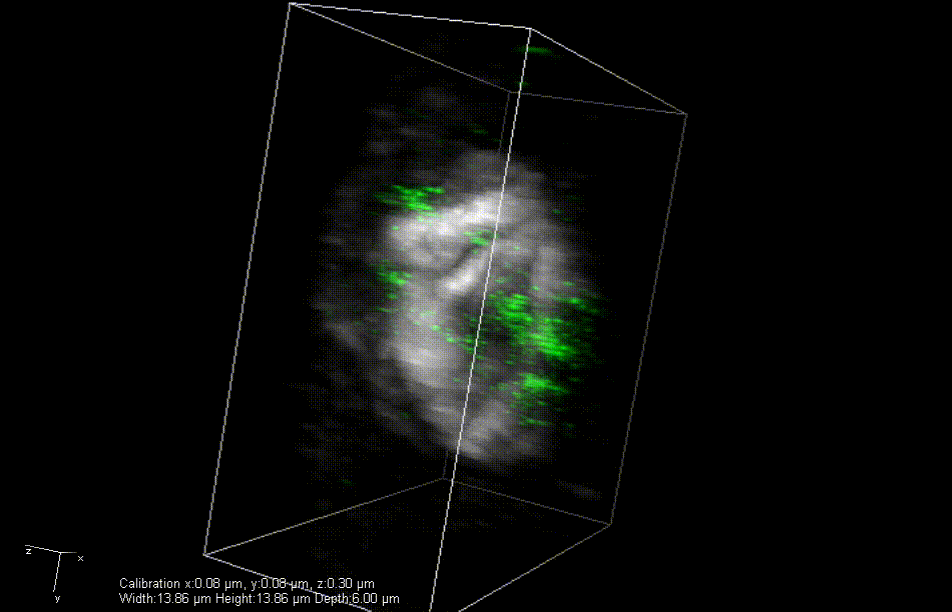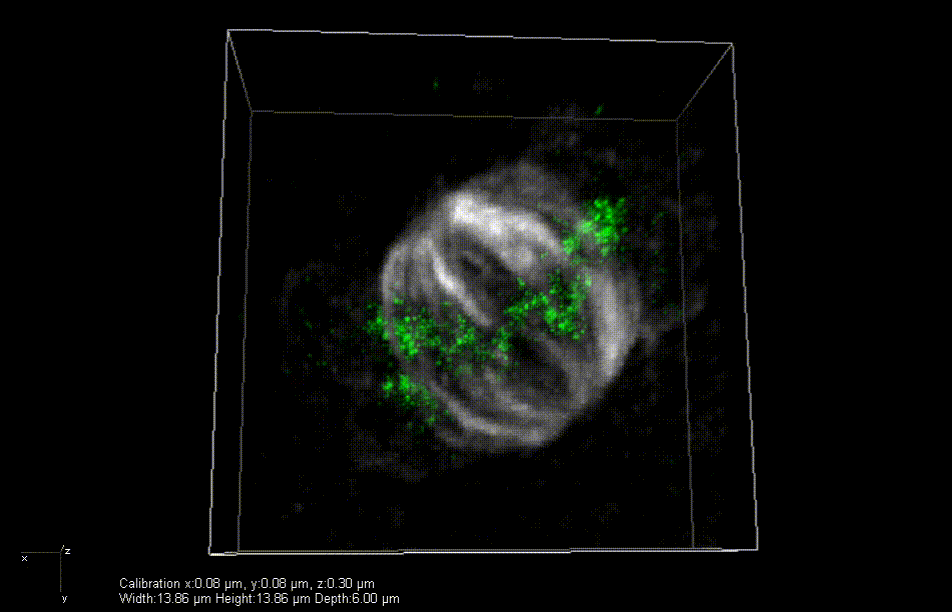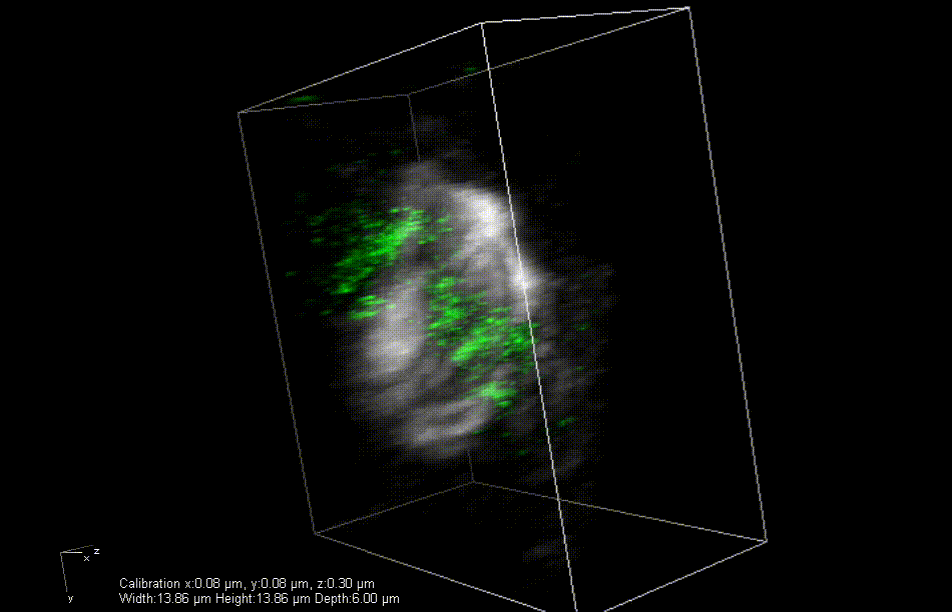
In mice engineered to lack the Lamin B2 gene, heart muscle cell chromosomes don’t link up properly (green) during cell division, preventing the spindles (gray) from effectively pulling the chromosomes apart. So, the cells get stuck together. Credit: Lu Han, Ph.D.
Even though heart muscle cell proliferation is abundant in newborn mice, eliminating Lamin B2 weakened their ability to regenerate heart tissue after injury. In contrast to eliminating the Lamin B2 gene, the researchers also used genetic engineering technology to overexpress the gene, making Lamin B2 more abundant in heart muscle cells. Once the mice had the extra Lamin B2, heart regeneration was restored.
Discovering the gene controlling heart muscle cell regeneration in mice was exciting, but researchers in Kühn’s lab wanted to make sure this mechanism applied to humans as well. They experimented with cardiomyocytes derived from human stem cells in a dish and with heart tissue collected from human infants during previous life-saving heart surgeries. When they eliminated Lamin B2 from these cells, they encountered the same cell division problems demonstrated in the mice. When they overexpressed Lamin B2, cell proliferation capacity was restored.

Bernhard Kühn, M.D. Credit: UPMC.
“This paper really nails a specific mechanism”, Kühn said. “Now that we know the mechanisms of how fully mature heart muscle cells are generated, other researchers can use this information to make mature heart muscle cells in a dish from stem cells.”
These new discoveries are essential for growing heart tissue in the lab to use in disease modeling and screening new drugs, Kühn said. More importantly, though, if scientists can successfully grow heart tissue outside of the human body, this tissue can be used in organ transplants so that human heart injury no longer has to be fatal.