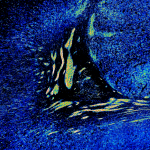
A University of Pittsburgh study, now published in Neurobiology of Disease, was first to show that a particular enzyme involved in many bodily functions, called NADPH Oxidase 2 (NOX2), is a major producer of oxidants in neurons that contribute to Parkinson’s disease progression.
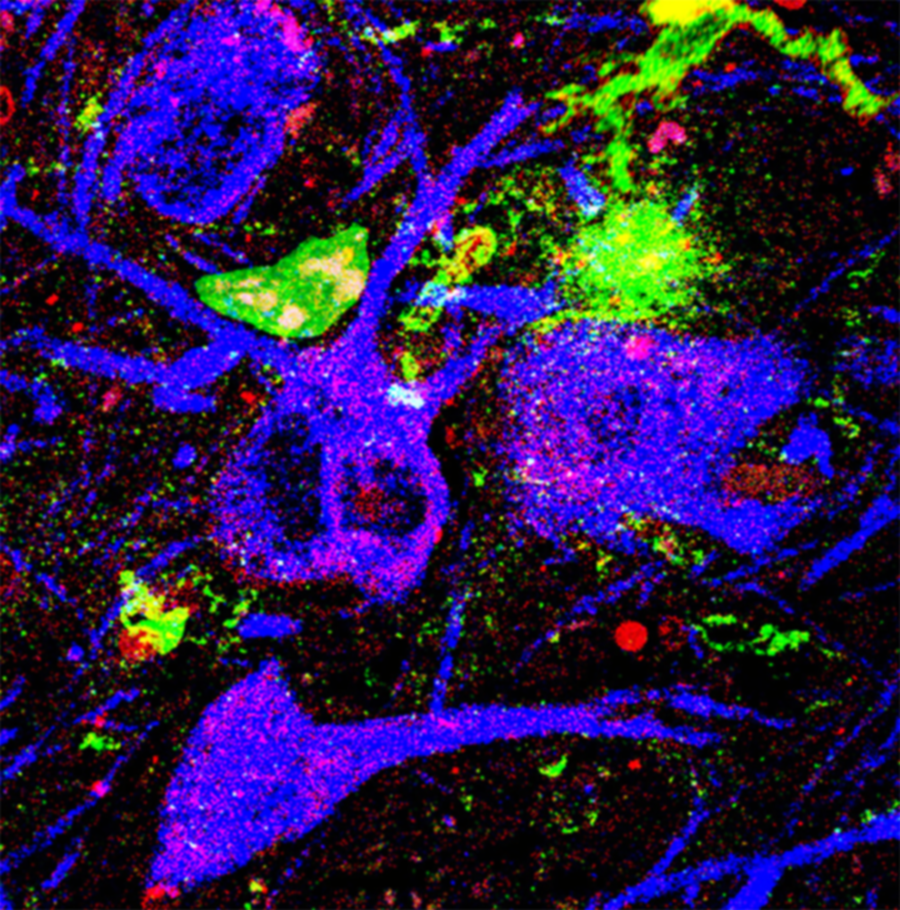
NOX2 (red) is highly active in dopamine neurons (blue) and microglia (green) of patients with Parkinson’s disease
Oxidative stress—or the accumulation of harmful free radicals called reactive oxygen species—accompanies our daily lives and our bodies need antioxidants from fresh foods to pacify it. But even though eating vegetables is good for our bodies, researchers do not think we can eat our way out of Parkinson’s disease. Instead, their discovery suggests that NOX2 inhibitors could play a therapeutic role against this neurogenerative disease that affects millions of people worldwide.
“Our strategy to limit damage to neurons in Parkinson’s disease is to block the enzyme responsible for oxidative stress instead of removing the reactive oxygen species themselves,” said Dr. Roberto Di Maio, the corresponding author of the study and assistant professor at the Pittsburgh Institute for Neurodegeneration (PIND). “Otherwise, we would need impossible amounts of antioxidants to get rid of the reactive oxygen species.”

Roberto Di Maio
Cells rely on a balance between oxidation and reduction to survive. When there is an imbalance favoring oxidation, reactive oxygen species interact with proteins or nucleic acids, changing their shape and function. If cells endure too much oxidative stress, they die.
Parkinson’s disease is an incurable neurodegenerative disease that robs people of their mobility and autonomy. For the 1 million people in the U.S. living with Parkinson’s disease, medications can mitigate symptoms, like tremors, but there are no treatments that prevent the death of the dopamine-producing neurons in the movement-controlling region of the brain called the substantia nigra pars compacta. These neurons are vulnerable to reactive oxygen species, which are products of oxidative stress.
“Many people have reported that oxidative stress goes awry in Parkinson’s disease. We are particularly interested in understanding what causes oxidative stress, how it goes awry and how can we prevent it,” said lead author Matthew Keeney, a Ph.D. candidate at Pitt who started studying Parkinson’s disease during his undergraduate career.
NOX2 is an enzyme that generates large amounts of reactive oxygen species upon interacting with other proteins. To track NOX2 in neurons, researchers developed a new imaging assay, called a proximity ligation assay, that produces a fluorescent signal that can be seen under the microscope whenever NOX2 is active.

Matthew Keeney
Di Maio and colleagues are first to report that NOX2 is highly active in the neurons of Parkinson’s disease patients and animal models. The researchers observed a 10-fold increase in NOX2 activity in the brain tissues of Parkinson’s disease patients compared to healthy patients’ samples. This 1,000% increase in NOX2 activity causes a massive increase in reactive oxygen species that quickly overwhelms the cell’s antioxidant defenses, leading to oxidative stress and cell death.
Encouragingly, blocking NOX2 reversed the oxidative stress in animals with Parkinson’s disease and prevented toxic buildup of alpha-synuclein, a key marker of Parkinson’s disease progression.
Di Maio and colleagues at PIND are now aiming to see if other NAPDH oxidase enzymes may have a role in Parkinson’s disease progression and if developing novel drugs to specifically inhibit them may improve patient outcomes.
There is no doubt that a moderate diet rich in fibers and natural antioxidants may be one of the many protective factors against the onset of many diseases, including neurodegenerative disease. But, unlike the cartoon Popeye, who can achieve super-human strength and invincibility by simply gulping down a can of spinach, we cannot overcome the effects of oxidative stress in the same way.
“If we can understand the causes of oxidative stress and how they affect downstream targets that lead to Parkinson’s disease, we can find novel targets to prevent it,” Di Maio said. “We think NOX2 might be a very appealing target for future therapeutic studies.”
Aaron Johnson is a Ph.D. candidate in the University of Pittsburgh School of Medicine’s Department of Pharmacology and Chemical Biology. He is participating in the UPMC Science Writing Mentorship Program.