University of Pittsburgh neurobiologists received a National Institutes of Health (NIH) Director’s Transformative Research Award to lead an ambitious $9 million, five-year multicenter effort to identify the molecular and genetic mechanisms that cause amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease.
This project has the potential to not only enhance understanding of ALS and a related disorder called frontotemporal lobar degeneration (FTLD), but also to identify genetic targets that could be treated with new gene therapies, said principal investigator Christopher Donnelly, Ph.D., assistant professor of neurobiology and scientific director of the LiveLikeLou Center for ALS Research at Pitt’s Brain Institute.
“ALS and FTLD are two fatal neurodegenerative conditions with no current treatment to prevent, slow or stop brain cell death, and some patients develop both disorders,” Donnelly said. “We think that studying their common biological pathways will help us find solutions for both disorders more quickly.”
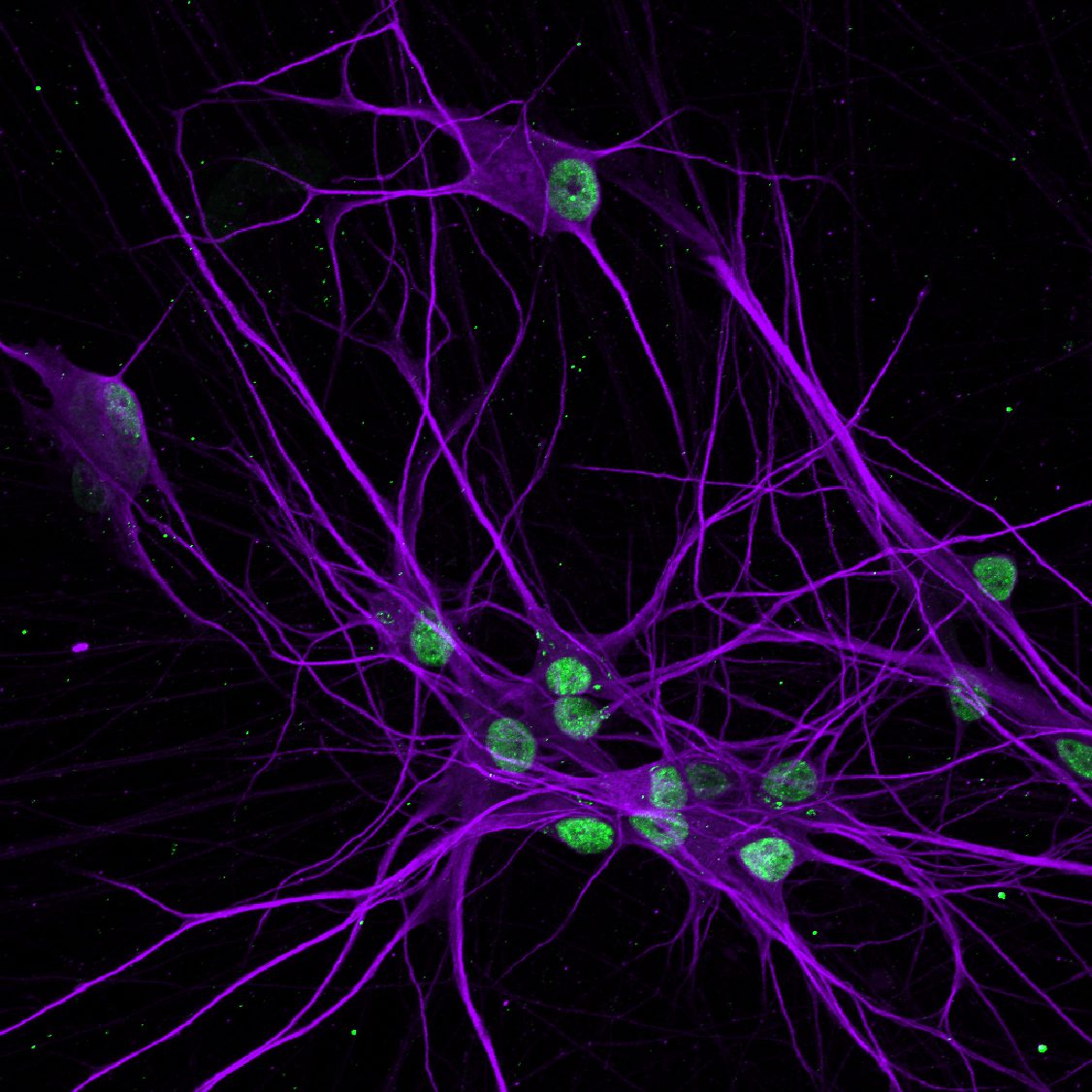
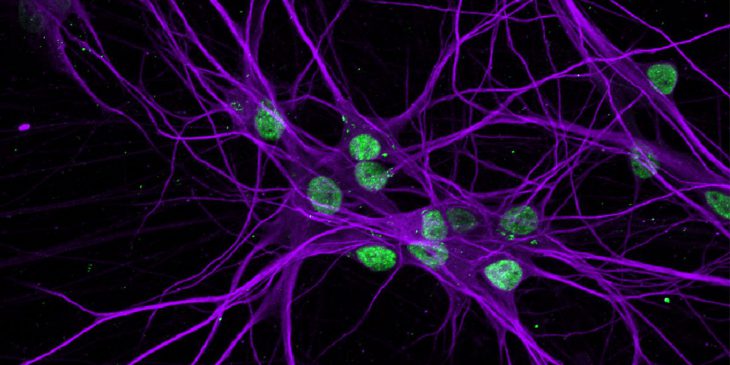
Pitt researchers collect skin cells from patients with ALS/FTLD and revert them into induced pluripotent stem cells, which can then be reprogrammed to become neurons for lab studies.
Up to 50 percent of ALS patients, in which muscles progressively weaken, eventually develop the dementia symptoms of FTLD. About 15 percent of patients are eventually diagnosed with both.
Previous research shows that abnormal clumping and distribution of a cellular protein called TDP-43 is behind 98 percent of ALS cases and 50 percent of FTLD diagnoses. But the genetic triggers for TDP-43’s dysfunction are not yet known.
The new effort aims to identify those disease-causing gene variants with the ultimate aim of developing effective therapies for ALS and FTLD. First, researchers at Pitt and the Mayo Clinic in Jacksonville, Florida, will collect samples of brain and spinal cord tissue from people who had ALS, FTLD or both when they died. The samples will be assessed at the single-cell level to develop genetic and cellular profiles. The findings from millions of these cells will then be integrated into first-of-their-kind data sets by researchers at the Massachusetts Institute of Technology and the Broad Institute in Cambridge, Massachusetts, to determine patterns of gene and protein alterations, which will be linked to the patients’ disease signs and symptoms.
“This process will show us where we should target interventions, such as gene therapies,” Donnelly said. “Then we can test those ideas in both cells and animal models of ALS and FTLD.”
The program is funded through an NIH effort to catalyze scientific discovery in which researchers are encouraged to think “outside the box” and pursue trailblazing ideas. This funding cycle included special-focus awards for ALS research.
“The science put forward by this cohort is exceptionally novel and creative and is sure to push the boundaries of what is known,” said NIH Director Francis S. Collins, M.D., Ph.D., in a press announcement. “These visionary investigators come from a wide breadth of career stages and show that groundbreaking science can happen at any career level given the right opportunity.”
Other project leaders include David Lacomis, M.D., and Julia Kofler, M.D., of Pitt and UPMC; Veronique Belzil, Ph.D., and Dennis Dickson, M.D., of the Mayo Clinic; Myriam Heiman, Ph.D., of MIT; and Manolis Kellis, Ph.D., of the Broad Institute.
The Donnelly and Belzil labs also receive funding for ALS research from the LiveLikeLou Foundation, which was founded in Pittsburgh.