Romanian prince Vlad III of Wallachia, popularly known as Vlad Dracula, is famous for his aversion to sunlight – a trait that gave rise to vampire legends. Like many myths, vampirism is partly based on fact; specifically, a rare blood disorder called porphyria. Although Vlad aggressively avoided exposure to sunlight, it wasn’t due to fear of spontaneous combustion but rather a hypersensitivity of the skin to sunlight, causing fluid-filled sacs or lesions, skin discoloration and scarring, among other deformities.
King George III, whose reign was later than Vlad Dracula, famously suffered from episodic madness, recently ascribed to porphyria. Astonishingly, more than two centuries later, this group of diseases called the porphyrias still have no cure.

Anu Balogun
I am a Ph.D. candidate working on signaling pathways in liver diseases in Dr. Kari Nejak-Bowen’s lab in the University of Pittsburgh Department of Pathology. Current treatment for porphyria focuses on providing relief for symptoms associated with acute attacks. Two of these treatments, Hemin and Givosiran®, are extremely expensive at over $100,000 and $500,000 annually, and porphyria is a significant healthcare burden in the U.S. These reasons motivated me to develop novel therapies for patients with porphyria.
Recently, I presented my research on a novel therapeutic strategy for porphyria at the American Association for Investigative Pathology at the final Experimental Biology meeting in Philadelphia, Pennsylvania.
Heme is important!
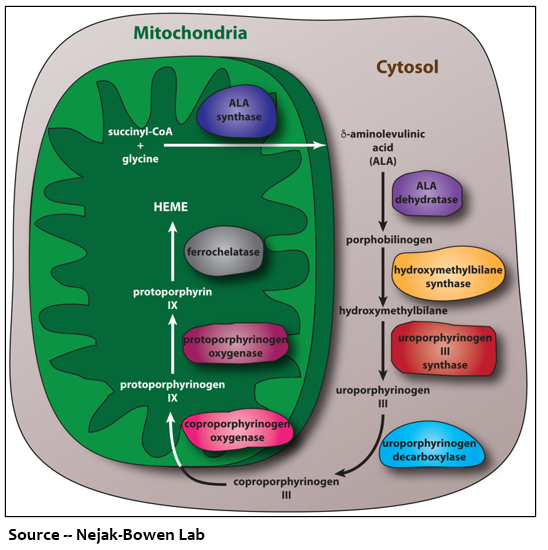
Understanding porphyria starts with heme, one of the body’s most essential and ubiquitous molecules. The unique structure of heme allows it to “host” an iron molecule, giving blood its red color. Heme is a component of different biological structures, including hemoglobin and myoglobin, and it is responsible for carrying oxygen across organs and tissues. Heme is synthesized in a series of eight steps, each controlled by different enzymes. Synthesis of heme begins in the mitochondria, the cell’s energy production hub, where the first enzyme creates the first porphyrin. This porphyrin then moves into the cytosol, or fluid inside the cell, where the second enzyme converts it to the next porphyrin. This process continues like a merry-go-round to produce heme. Approximately 80% of heme is made in cells located in the bone marrow and 15% is produced in liver cells.
What causes the porphyrias?
Defects in the eight enzymes involved in the heme-producing pathway result in buildup of porphyrins and their precursors, causing porphyria. When porphyrins accumulate in the liver, this disease is called hepatic; if they build up in the bone marrow, it is considered erythropoietic. Clinically, the porphyrias are classified in two ways. Acute porphyrias primarily affect the nervous system manifesting as hallucination, disorientation, seizures, difficulty breathing and paralysis, among other clinical symptoms. Chronic porphyrias mainly present as sensitivity to light, blisters, infections, scarring, hyperpigmentation and other skin conditions. The porphyrias are genetic but can also be triggered by medications, recreational drugs, smoking, alcohol use, viral diseases, and menstrual hormones, all of which can force the body to produce excess porphyrins. These symptoms and triggers demonstrate why a one-size-fits-all treatment cannot easily be applied to the porphyrias.
Porphyrias –the traffic jam situation
I like to describe this disease using a traffic jam analogy. In this scenario, the street is the heme biosynthesis pathway, the cars are the porphyrins, and the traffic light represents the enzymes. When everything is working correctly, each car (porphyrin) arrives at a red traffic light (enzyme) and is converted to the next porphyrin. The green light comes on, and the car proceeds. The process goes around and around, generating heme. However, when there is a defect in the traffic light, the light remains red, and the car (porphyrin) is unable to be converted, causing a traffic jam where the other cars (porphyrins) pile up behind it.
One solution may be to contact the “traffic cop” who controls the traffic lights. So, where can we find this mysterious person?
Are signaling pathways the mysterious traffic cops?
Because the porphyrias have a vital liver component, my team thought that the Wnt signaling pathway, which keeps critical functions in the liver at optimal performance, might be acting as the traffic cop remotely controlling the traffic jam situation in porphyria. When a molecule known as a ligand attaches itself to a receptor component of the Wnt pathway, it causes a cascade of events that leads to increased activation of a protein called beta-catenin. Mutations or overproduction of beta-catenin or other dysregulation in the Wnt pathway is associated with many diseases.
Simulating porphyria in mice
When we feed mice an additive called 3,5-Diethoxycarbonyl-1,4-Dihydrocollindine (DDC) as part of their diet, they develop porphyria like humans. To test our hypothesis that beta-catenin contributes to the traffic jam, we fed mice DDC and then treated them with a Wnt inhibitor to see if their symptoms would resolve.
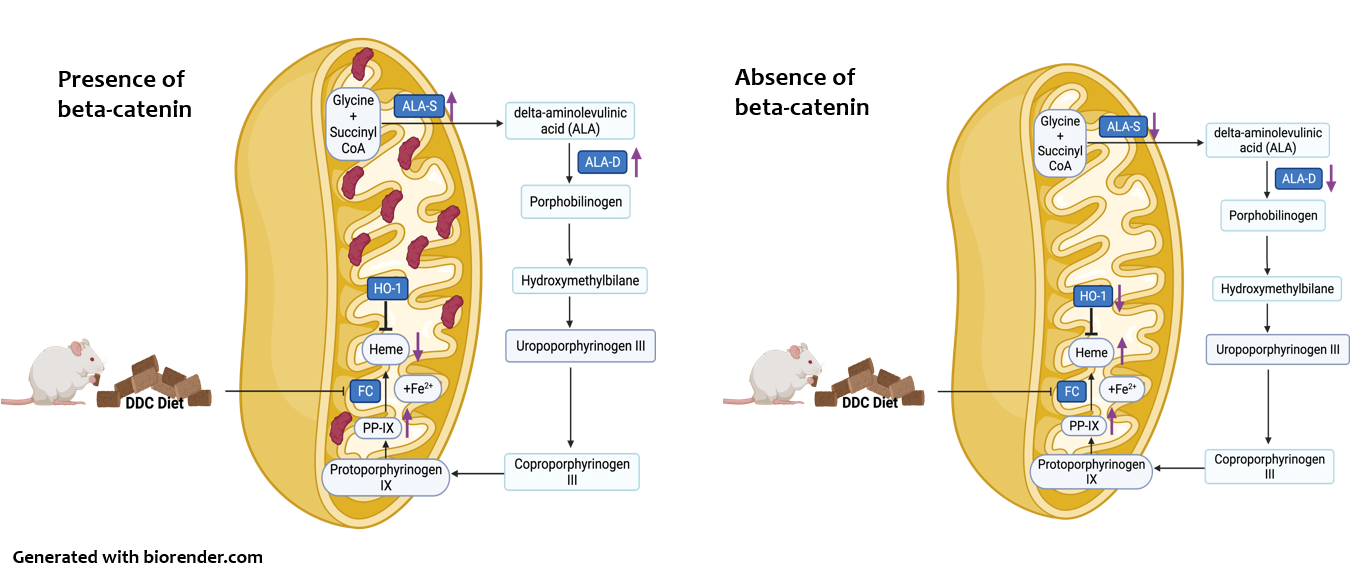
What happens when the traffic cop is removed?
After we inhibited beta-catenin in DDC-fed mice, we found that liver injury and accumulation of porphyrins was significantly reduced. The car jam unblocked, freeing up the heme biosynthesis pathway and allowing all the cars to go through and produce heme efficiently. We also found that heme enzymes were reduced, and heme levels were preserved. We think that the traffic cop beta-catenin is specifically reducing these enzymes. The absence of beta-catenin also fine-tuned a process called autophagy that acts as a cellular recycling system. Removing beta-catenin allows cells to clear accumulated porphyrins like a tow truck removing cars stuck in a traffic jam.
The Conclusion
Inhibiting beta-catenin ensures heme enzymes produce heme at optimal levels, prevents the accumulation of porphyrins that can injure the liver, and clears accumulated porphyrins. We find this exciting as several Wnt pathway inhibitors are being tested on other diseases in clinical trials. With this strategy, we hope to provide a cost-effective treatment for porphyria patients that address this disease in more than one way.
Anu Balogun is a Ph.D. candidate in the University of Pittsburgh School of Medicine’s Department of Pathology. She is participating in the UPMC Science Writing Mentorship Program.