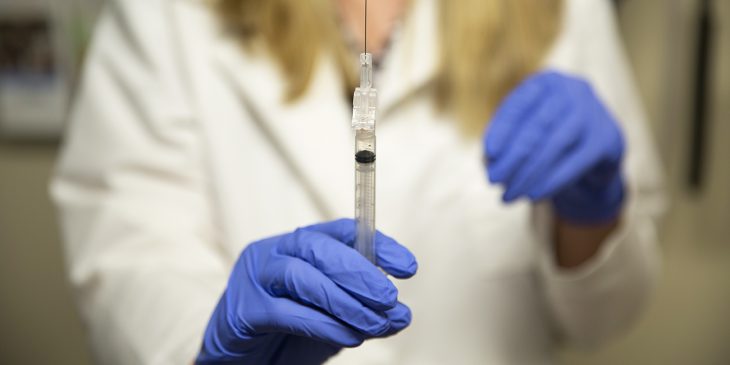
The vast majority of the flu vaccines administered by UPMC clinicians to patients and employees will be egg-free this season, with the goal of providing better protection against the deadly virus.
UPMC will be offering Flublok or Flucelvax to all patients age 4 or older throughout the 2018-2019 flu season, and all of its 85,000 employees. Neither vaccine contains egg proteins, antibiotics, preservatives or live virus, and they come in single-dose, prefilled syringes that do not contain natural rubber latex. They both immunize against the four strains of flu that world health officials forecast as most likely to circulate.
“These vaccines are superior to the traditional, egg-based vaccine in people old enough to receive them,” said Dr. Richard K. Zimmerman, advisor to the UPMC Influenza Committee and director of PittVax, the University of Pittsburgh’s vaccination research group, which is funded by the U.S. Centers for Disease Control and Prevention to study flu vaccine effectiveness. “As last season’s particularly devastating and deadly flu epidemic demonstrated, it is crucial that we continue to improve flu vaccine and adopt better technology to produce it.”
Most of the world’s supply of influenza vaccine is made in eggs, a 70-year-old manufacturing process that was originally adopted because fertilized eggs were an inexpensive vessel in which to grow the virus. But that process also gives rise to mutations that can make the vaccine less effective. Such mutations made the flu vaccine only partially protective against the H3N2 strain of the virus that predominated the last two years.
Benefits of the egg-free vaccine include:
– It is not made with eggs, so it is safer for people with egg-related allergies.
– Scientific studies have found it to be as much as 30 percent more effective at offering protection against the flu than the egg-based vaccine.
– Studies have also shown it to provide better protection in seniors, so UPMC will offer it in place of high-dose flu vaccination for those age 65 or older.
“UPMC is the only health system in Pittsburgh to make the Immunization Action Coalition’s Influenza Vaccination Honor Roll,” said Mark Tanis, director of both UPMC Work Partners Employee Health Services and UPMC’s Influenza Committee. “We’re committed to preventing flu in our patients, employees and community – and offering the most effective flu vaccines is one of the best ways to do that.”
Flublok uses a recombinant DNA technique whereby manufacturers isolate a protein from the flu virus, combine it with portions of another virus and grow it in cells. The flu protein is then harvested from the cells, purified and packaged in a vaccine. It is approved by the U.S. Food and Drug Administration (FDA) for people age 18 and older.
Flucelvax uses a process that puts the flu virus into cultured cells and allows it to replicate. The virus-containing fluid is harvested, and the virus antigen – the part of the virus that triggers the human immune system to respond – is purified and placed in the vaccine. It is FDA-approved for people older than 4.
UPMC will offer the egg-based FluZone to patients age 6 months to 4 years because the egg-free versions haven’t yet been approved by the FDA for people this young.
Flu vaccine shipments are arriving at UPMC facilities, and the health system expects to begin offering vaccines by the second week of September. Patients are encouraged to contact their primary care physicians or visit the most convenient UPMC Urgent Care clinic to receive a flu shot.