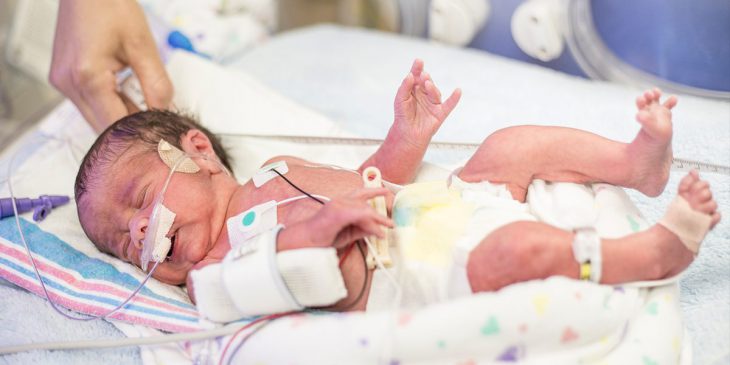
In utero, an extra blood vessel called patent ductus arteriosus, or PDA, allows blood circulation to skip the lungs of a developing baby. Normally, either before birth or shortly thereafter, the vessel shrinks and closes.
But sometimes, particularly in premature infants, the PDA is large and more likely to stay open, which may require the infant to be put on mechanical ventilation and lead to problems that strain and enlarge the child’s heart. The best course of action to help the newborn – medication, insertion of a catheter or corrective surgery – isn’t always obvious.

Dr. Wendy King
To help doctors provide the best possible guidance to parents, the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) has selected the University of Pittsburgh as the Data Coordinating Center for a multicenter, randomized trial titled “Percutaneous intervention versus observational trial of arterial ductus in lower gestational age infants,” or PIVOTAL. Dr. Wendy King, associate professor of epidemiology at Pitt’s School of Public Health, and Dr. Stephen Wisniewski, vice provost at Pitt and co-director of Pitt Public Health’s Epidemiology Data Center, are principal investigators on the trial.
“Using the best data science methods is critical to answering clinical questions, which in this trial could prevent chronic lung disease, intestinal injury, brain damage, congestive heart failure and even mortality among preterm infants,” King said.
In preterm infants on breathing support with PDAs that affect the flow of their blood, the trial will compare cardiopulmonary outcomes, such as days free of mechanical ventilation, following PDA closure via a minimally invasive heart catheter closure-device versus supportive care without closure. The study also will evaluate safety and improvement in neurodevelopmental outcomes.
The Data Coordinating Center at Pitt will support the study design and protocol development, provide data management, conduct statistical analyses, facilitate communication and coordination across the clinical sites, and collaborate in the preparation and dissemination of study results. Nationwide Children’s Hospital in Columbus will serve as the trial’s Clinical Coordinating Center, developing the scientific rationale for the trial and managing subject recruitment at all sites, application of the trial intervention, outcomes follow-up and dissemination of results.
In 2019, the U.S. Food and Drug Administration approved a device to close PDA in preterm infants. But the long-term effects on patient outcomes remain unknown. Historically, invasive surgery was the only option to close a PDA if medication was unsuccessful. The new device offers a less invasive approach using a catheter inserted into a leg vein.
Abbott, the manufacturer of the device, is also contributing funding to the NIH-sponsored trial.