Researchers at the University of Pittsburgh have found a way to observe how misbehaving mitochondria can lead to neurological problems resembling neurodegenerative diseases in humans.
The study, published recently in eLife, uses genetically-modified zebrafish larvae, whose see-through bodies give researchers a chance to watch what’s happening inside their nervous system when mitochondria break down, such as during Parkinson’s, Alzheimer’s, Huntington’s and chronic traumatic encephalopathy (CTE).
Edward Burton, M.D., Ph.D., associate professor of neurology at Pitt, UPMC Endowed Chair of Movement Disorders and neurologist at UPMC, and colleagues caused mitochondrial damage in specific compartments of zebrafish neurons, using technology developed by Marcel Bruchez, Ph.D., at Carnegie Mellon University.
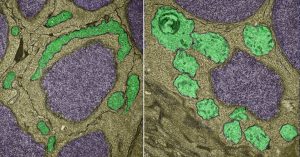
Normal mitochondria (left, green) and light-damaged mitochondria (right)
Burton and colleagues genetically-modified zebrafish to express a protein called dL5 in the mitochondria of their neurons. The dL5 protein acts as a receptor that binds fluorescent molecules, which emit harmful oxygen molecules when exposed to red light, causing oxidative damage to the mitochondria.
“The really big first here is that we’ve got a way of targeting a one micrometer component of specific cells in a whole animal, with absolute precision in terms of where and when the damage happens and how much damage there is,” Burton said. “Compare that to coarser techniques, such as adding chemicals to the zebrafish’s water — there’s no way to control which cells get damaged.”

Zebrafish with damaged mitochondria in their nervous systems (left) don’t move around like normal zebrafish (right).
The researchers found that zebrafish with damaged mitochondria in their nervous system had no motor responses to stimulation. The mitochondria of affected zebrafish were swollen, losing many of their signature folds, in which ATP production— the main energy units used by cells — takes place. Without ATP, the neurons lost their membrane stability and started to die about a day after the initial light-evoked damage.
Zebrafish and human brains share many of the same structures and neuronal populations affected in neurodegeneration, such as dopamine-producing neurons that die in Parkinson’s disease. By causing damage to the mitochondria of living zebrafish and observing what happened next, Burton and his team were able to glean insights into what might happen in humans with damaged mitochondria.
“I look after Parkinson’s disease patients clinically at UPMC, and from a clinician’s point of view it’s very frustrating because we can only treat their symptoms,” Burton said. “We know they have damaged mitochondria. What I really need is a treatment that targets downstream events, to prevent the pathogenic cascade and arrest or slow the progression of clinical symptoms.”
Burton and his lab have now restricted the expression of dL5 to only dopaminergic neurons in the brains of their zebrafish. Over the next one to two years, they expect to create a timeline of the biochemical events underlying cell death that mimics the one found in human patients.