A team of University of Pittsburgh neuroscientists and computational biologists have moved another step toward preventing brain cell death in the shadow cast by an acute stroke. In a study published this week in the Proceedings of the National Academy of Sciences, they describe a first-in-class molecule that stops a key protein-protein interaction from opening the door to stroke-triggered damage to neurons.
A stroke occurs because a clot clogs a brain blood vessel, blocking blood and oxygen from reaching neurons and killing them, explained Elias Aizenman, PhD, senior investigator and professor of neurobiology at Pitt School of Medicine. But even if the clot is rapidly dissolved with medication or removed with a catheter, a technique pioneered by UPMC specialists, neurons in the areas neighboring the stroke can still become dysfunctional and die hours or days later, even when their blood and oxygen supply might not have been interrupted.
“We have been trying to find ways to prevent this cell death cascade, which occurs in an area called the stroke penumbra,” Aizenman said. “This could also help patient recovery from strokes in which the clot is in an inaccessible blood vessel or if the patient could not get to the hospital in time for early intervention.”
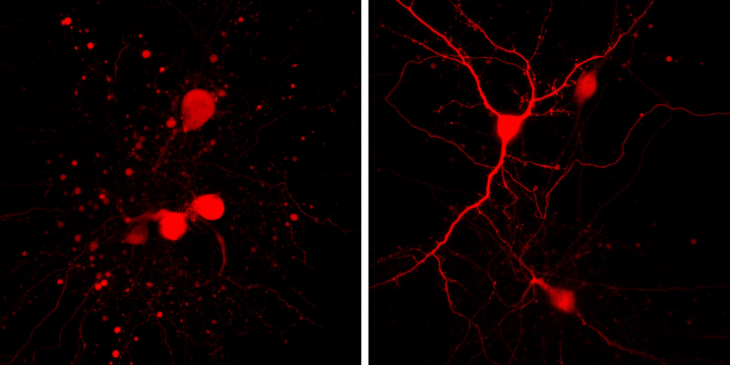
In a study published two years ago in the Journal of Neuroscience, Aizenman and team showed that the cell-death specific interaction between a potassium channel and a protein called syntaxin led to the increased activity of new channels or gateways in the neuronal cell membrane that encouraged potassium ions to leave the neuron, setting the stage for a process of cell death called apoptosis. They showed that that cell death could be prevented by interfering with the interaction of syntaxin with the potassium channel with a blood brain barrier-permeable peptide, but the agent they used is not a favored form for a drug.
They built on that work in the current project by discovering a small molecule called cpd5 that has a similar impact on syntaxin’s activity and could lead to further agents that have the potential to be made into drugs. Part of the challenge was finding the spot were syntaxin and the potassium channel, called Kv2.1, could engage each other – and then blocking that site to prevent the interaction.
“We used molecular modeling, biophysics and the experimental cellular validation to predict where and what that binding site could be,” said co-senior author Carlos Camacho, PhD, associate professor of computational and systems biology at Pitt. “And then we tested 28 million compounds using a computer model of the interaction and found six that had potential to be neuroprotective.”
For the PNAS study, they tested cpd5 in cell experiments and found that it did indeed block syntaxin and Kv2.1 interaction and consequent potassium ion outflow and cell death. The research team is now examining cpd5 in animal models of stroke to further gauge its neuroprotective effects. The researchers consider cpd5 as a first-in-class compound that will spark the development of more potent agents that could one day be used in stroke treatment.
“Our hope is that neuroprotective agents like this could also be of help in the treatment of chronic, progressive neurological illnesses, such as ALS, Alzheimer’s disease and Huntington’s disease,” Aizenman said. “We are starting to test that, too, in fruit fly and other experimental models using genetic and molecular approaches to prevent the critical Kv2.1-syntaxin interaction.”
The co-lead authors of the paper were Chung-Yang Yeh and Zhaofeng Ye, graduate students in the Aizenman and Camacho laboratories. This work was supported by National Institutes of Health Grants NS043277, GM097082, and DC007905.
ABOUT THE AUTHOR: Anita Srikameswaran, MD, is the program director at the University of Pittsburgh Brain Institute.