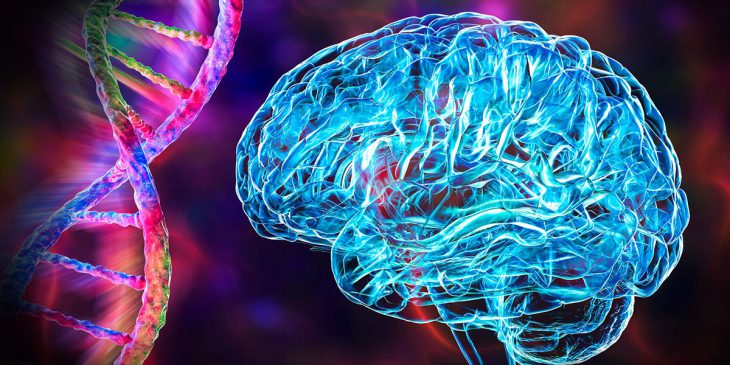
A rare but potentially debilitating brain disorder finally has a definitive cause, thanks to research teams working on opposite sides of the globe.
A mutation in the gene that manages the transportation out of cells of zinc, an essential dietary micronutrient, is responsible for the disorder, called hypomyelinating leukodystrophy. The research, jointly led by Dr. Quasar Padiath at the University of Pittsburgh and Dr. Anju Shukla at the Kasturba Medical College in India, is reported in the journal Brain.

Quasar Padiath, Ph.D.
This is the first time that a mutation in a zinc transporter gene – in this case, TMEM163 – has been definitively linked to the development of any brain disorder, and it has the potential to provide insights into the role of zinc in normal brain development, injury and disease.
“Discovering a new gene responsible for causing a disease is always exciting; that feeling never gets old,” said Padiath, associate professor of human genetics and neurobiology at Pitt. “And finding out that a zinc transporter is really important for proper myelin development could have many clinical implications and offer new ways of treating other related neurological conditions.”
Hypomyelinating leukodystrophies are rare and often fatal neurological disorders caused by defects in genes involved in growth or maintenance of myelin, the fatty layer of insulation surrounding nerves that helps them transmit electrical impulses. As the myelin layer gradually gets thinner and is lost in these patients, nerve signals slow to a crawl, ushering in a slew of neurological problems, including impaired movement and balance control, muscle wasting, problems with vision, and hearing and memory loss.
While genes have been linked to leukodystrophies, the genetic underpinnings for the majority of cases are still unknown. To identify the root cause of a patient’s condition and recommend the most appropriate therapy, clinical neurologists often turn to researchers like Padiath.
By combing through patients’ genomes, Padiath looks for mutations and analyzes the effect of these mutations in cells and animal models, such as mice. Such an analysis is no small feat. To definitively link a new gene mutation to disease symptoms, multiple independent patient cases that share the same gene defect and clinical presentation have to be identified.
For rare diseases, such as hypomyelinating leukodystrophies, finding such cases is possible only by tapping a network of scientific and clinical collaborators from all over the world. In this study, the first patient sample came from Shukla, a professor of medical genetics at Manipal in southwest India. Inquiries to other groups in the U.S. and the Netherlands identified additional families who also carried mutations in the same gene.
A series of in-depth lab studies showed that the TMEM163 mutations impair the transporter’s ability to effectively shunt zinc from inside the cell, causing reduced production of proteins responsible for synthesis and maintenance of myelin and increasing cell death.
“Understanding how genes cause rare diseases is the first step in the process of finding treatments,” said Padiath. “It is important to remember that diseases that are rare in the global context are very important and real for patients and their families. Studying these diseases helps find cures and give hope to patients and valuable insights into therapeutic targets essential for normal cell functioning.”
Michelle do Rosario, of Manipal, and Dr. Guillermo Rodriguez Bey, of Pitt, were the co-lead authors of the study. Additional authors can be found in the Brain article.