UPMC leaders today discussed the U.S. Food and Drug Administration’s recent expansion of the eligibility guidelines for COVID-19 monoclonal antibody treatment, as well as UPMC’s success in making this life-saving treatment widely accessible.
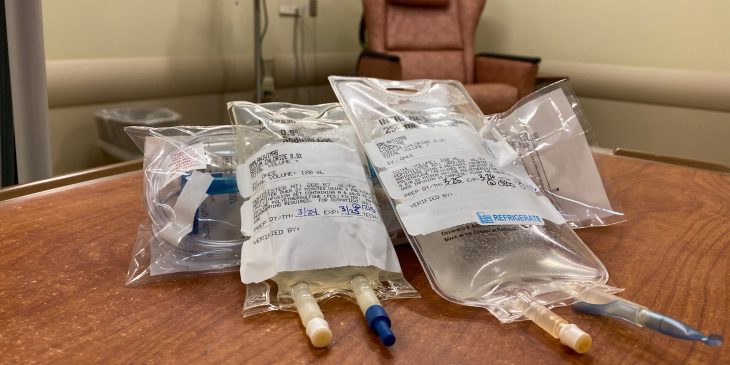
Monoclonal antibodies, which are given through a one-time IV infusion, are stronger versions of the body’s natural antibodies that destroy the virus quickly before it can cause harm.
The new FDA guidance allows these treatments to be provided to slightly overweight adults, former or current smokers, people with lung conditions or asthma, pregnant women, individuals with chronic diseases, such as diabetes, high blood pressure and kidney disease, patients with immunosuppressive conditions, and many others.
UPMC collaborated with the federal government to make monoclonal antibodies available to all patients and to quickly adopt the FDA’s expanded eligibility criteria, noted Dr. Meredith Chuk, the allocation, distribution and administration lead, Health and Human Services Office of the Assistant Secretary for Preparedness and Response.
“We thank our partners at UPMC for modeling how to make this therapy widely available and educating providers and patients so that we can maximize this treatment for the most vulnerable communities,” said Chuk.
UPMC has seen a 25-fold increase in the administration of monoclonal antibody treatments since March. They are available at 46 UPMC locations, including in dedicated infusion centers and every UPMC emergency department. It has also been brought to patients’ homes and administered in long-term care facilities and behavioral health centers.
“If you are diagnosed with COVID-19, even if your symptoms are mild, even if you are vaccinated and happen to get a breakthrough infection, ask about monoclonal antibodies,” said Tami Minnier, UPMC’s chief quality officer. “There’s a good chance you’re eligible, and they work best the sooner you get them.”
Dr. Steven Evans, a surgical oncologist at UPMC Hillman Cancer Center, emphasized UPMC has mitigated racial disparities in its administration of this life-saving treatment through increased information, access and discussion with Black and Brown patients and community members.
“At the very beginning of UPMC’s experience with monoclonal antibodies, people of color were receiving this treatment at a much lower rate. UPMC saw this gap and immediately began a multi-pronged approach to improve access – drawing heavily from our success with improving access to COVID-19 vaccinations,” explained Evans.
Dr. Donald Yealy, chief medical officer at UPMC, described UPMC’s commitment to “learning while doing” through ongoing clinical trials related to monoclonal antibodies. UPMC launched the OPTIMISE-C19 trial to analyze whether one of the approved treatments is more effective than the other.
“As new monoclonal antibodies are introduced, we’ll include them in this trial. This is possible because of our tremendous local expert teams working closely with our federal partners,” said Yealy. “Together we share a commitment to promoting evidence-based treatments with both rigor and speed – not one or the other.”
UPMC’s expanded administration and continued research surrounding this treatment option and COVID-19 vaccinations may help those most vulnerable to the virus move a step closer to normalcy. To learn more about the monoclonal antibody treatments available at UPMC, visit upmc.com/antibodytreatment.