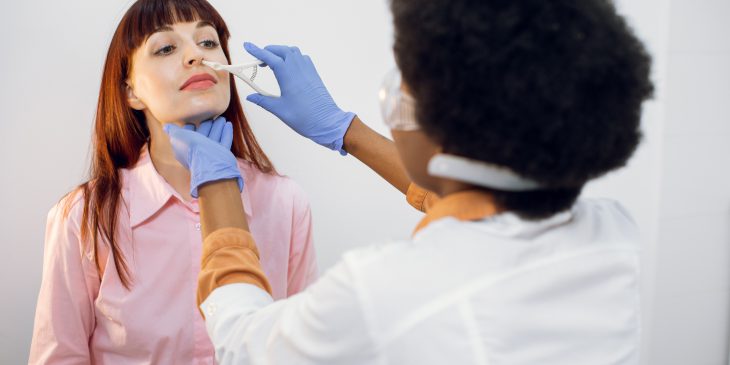
Some cystic fibrosis patients don’t really know what it feels like to have a runny nose — until now. A new study from a University of Pittsburgh research team shows that the drug Trikafta decreases nasal congestion for these patients and thins out nasal secretions, helping clear the sinuses.
Now published in the Journal of Cystic Fibrosis, the study reports that Trikafta — previously shown to improve lung function — also improved sinus and nasal symptoms in 90% of patients enrolled in a small clinical trial.

Dr. Anna Zemke
“People with cystic fibrosis often have a blocked nose, headaches, facial pain and a postnasal drip,” said senior author Dr. Anna Zemke, an intensivist at UPMC and assistant professor in the Division of Pulmonary, Allergy & Critical Care Medicine in Pitt’s School of Medicine. “When patients took the drug, some said, ‘I’ve never had a runny nose in my life. Why is my nose running so much?’ We think that’s because they are so used to feeling clogged up; that’s what normal is for them.”
Although blowing the nose more often might be uncomfortable for patients on Trikafta, a runny nose is a sign that the sinuses are draining better.
Cystic fibrosis, or CF, is a genetic disorder caused by a mutation in the CFTR gene, which leads to build up of mucus that clogs airways, lungs and sinuses. Bacterial infection and chronic inflammation in the sinuses can lead to long-term disease complications, such as facial remodeling of their nasal bridge and infections that may require surgery. In the lungs, CF causes long-term damage that eventually leads to lung failure and death.
Until recently, the only long-term treatment for CF was lung transplant from a healthy donor. New lungs are often life-changing and life-extending for these patients, but a transplant doesn’t correct nasal and sinus symptoms.
In 2019, the U.S. Food and Drug Administration (FDA) approved a drug combination called elexacaftor-tezacaftor-ivacaftor (Trikafta) for CF patients who carry a CFTR mutation called F508del — about 90% of the CF population in the U.S. This landmark treatment, which corrects function of the defective protein encoded by the mutant CFTR F508del gene, has been shown to greatly improve lung function.
But less was known about whether Trikafta could also improve sinus symptoms in CF patients.
Using the aptly named SNOT scale, or SinoNasal Outcome Test, which measures sinus symptoms and quality of life, along with imaging of the nose and sinuses, Zemke and her team evaluated 34 people with CF between the ages of 12 and 60 before and after Trikafta treatment.
“Within a week of starting Trikafta, people’s symptoms of sinus disease improved almost to the level of the general population, and that improvement persisted after nine months,” said Zemke.
Symptoms of clogged nose, facial pain, headaches and postnasal drip nearly vanished for most patients.
According to Zemke, one of the most remarkable observations from the study was the decrease in nasal polyps, or non-cancerous growths, that obstruct airways in CF patients. Before the development of Trikafta, the only treatment for polyps was surgical removal, but the polyps eventually grow back.
“Everybody’s polyps got better with treatment, and many people’s polyps entirely disappeared,” she added. “This is the first non-surgical treatment for nasal polyps in CF.”
Patients also saw a decrease in sinus congestion with Trikafta treatment.
“Before treatment, participants’ sinuses were filled with mucus,” said Zemke. “But after treatment, CT scans showed that 90% of patients had a big improvement. Their sinuses were filled with air instead of mucus.”
A common symptom of CF is loss of smell, which can make it hard to enjoy food or detect dangerous odors like smoke or a gas leak. Despite improvements in patients’ sinus function, their sense of smell didn’t get better, Zemke was surprised to find. She said it might be due to permanent nerve damage or because patients may need to re-train their brain to smell again.
In light of the recent FDA approval of Trikafta for CF patients aged 6 to 11, Zemke and her colleagues at Pitt and the University of North Carolina are now expanding their study to this younger group.
Aaron Johnson is a Ph.D. candidate in the University of Pittsburgh School of Medicine’s Department of Pharmacology and Chemical Biology. He is participating in the UPMC Science Writing Mentorship Program.