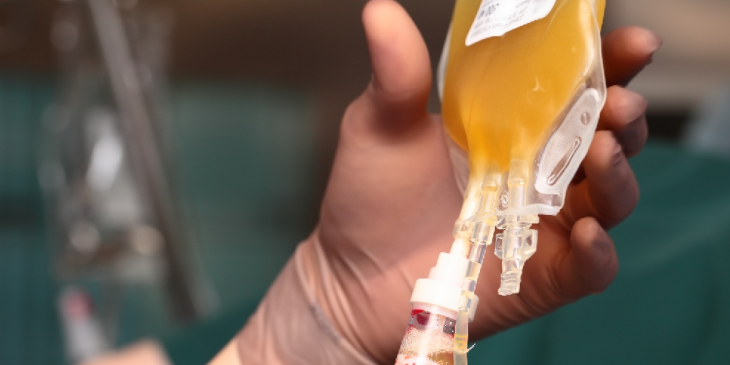
UPMC has enrolled in a national clinical trial hoping to provide patients with a potential therapy for COVID-19, called “convalescent plasma.” For this potential treatment, blood plasma containing antibodies against SARS-CoV-2, the virus that causes COVID-19, is taken from altruistic donors and given to patients battling the infection.
The trial, approved in early April by the U.S. Food and Drug Administration, is aimed at determining whether convalescent plasma therapy is effective for treating COVID-19 patients.
“It is a therapy that has been used in multiple viral outbreaks going back to the late 1800s with measles, and the 1918 flu pandemic, and used over many years, including even during the initial SARS 1 outbreak,” explained Dr. John McDyer, director of UPMC’s Lung Transplantation Translational Research Program and the principal investigator for UPMC’s clinical trial site. “The concept for using convalescent plasma falls under the idea of passive immunity. Individuals who don’t have immunity, get immunity from a source, like blood, that is given to them.”
The clinical trial hypothesizes giving convalescent plasma from donors previously infected with COVID-19 to patients currently fighting the novel coronavirus in an effort to build-up their immunity against it. The process of passive immunity suggests that infusing donor plasma from these patients would transfer antibodies—virus neutralizing proteins found in the plasma— to sick patients, bolstering their immune system to fight off the illness.
UPMC is vetting potential plasma donors by first screening them over the phone and then bringing them into the clinic for a medical screening.
The FDA requires the researchers to verify that potential donors have cleared the COVID-19 infection by administering a nasal swab test. Donors must be symptom-free for at least 14 days to decrease the likelihood they are still positive for the virus. Additionally, a blood test is conducted to ensure the potential donor has antibodies to the virus.
The donors come into the Falk Clinic, next to UPMC Presbyterian, and receive free testing. After the test results come back— which takes about two days— it is then determined whether the patient is medically cleared to be referred to Vitalant, the blood bank for Western Pennsylvania, for donation collection.
UPMC has already referred about a dozen recovered donors as of April 15.
“It is a true testament to Pittsburgh’s spirit of helping our neighbors that altruistic donors have already stepped up to give their plasma to help our hospitalized patients,” said Dr. McDyer, who is also an associate professor of medicine at the University of Pittsburgh. “We’re excited to enroll COVID-19 patients in our hospitals very soon.”
While McDyer is cautiously optimistic of the trial, he notes that there is much research to be done before establishing convalescent plasma as a key therapy for COVID-19. As of now, the treatment is being designated only to those hospitalized with the virus.
“It’s too soon to declare it as an effective therapy. It appears to be well tolerated and safe but there haven’t been large numbers of patients receiving it,” McDyer said. “This information is coming out by hour by hour, but the early signal is convalescent plasma should be relatively well-tolerated.”
UPMC is seeking people who tested positive for COVID-19 by the nasal swab test and have since recovered to be potential donors for the clinical trial.
Those interested in donating plasma should email COVID Convalescent Plasma Pittsburgh Project at C2P3@upmc.edu. Only those who have tested positive for COVID-19 and fully recovered are eligible to donate.