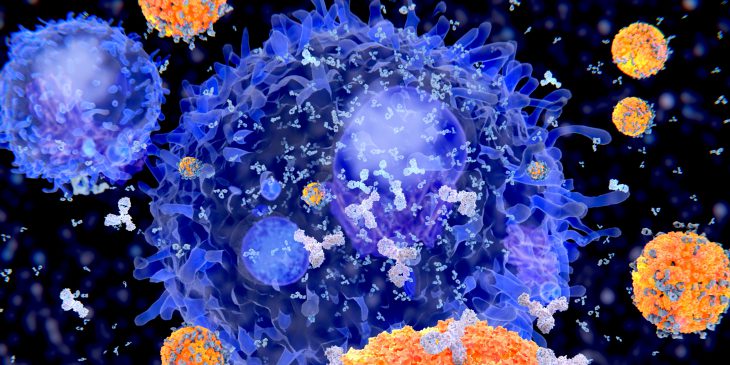
The immune system’s B cells have the unique distinction of being the only cells in the human body that evolve, in real time – and an international team of scientists today announce in Nature Immunology that they have uncovered new insights into how these cells rapidly evolve in response to an infection or vaccine to produce better protective antibodies.

Dr. Harinder Singh
The fundamental discovery could lead to therapies that prepare our bodies to fight deadly diseases or, on the flip side, prevent our immune system from attacking transplanted organs. The research was funded in part by the UPMC Immune Transplant and Therapy Center – a partnership between UPMC and the University of Pittsburgh dedicated to understanding and harnessing the power of the immune system.
“It is utterly fascinating, the way B cells evolve in mere weeks to become stronger magnets to a newly invading pathogen, seeking out and capturing viruses and bacteria for destruction,” said co-author Dr. Harinder Singh, professor and director of systems immunology in Pitt’s School of Medicine. “By gaining a deeper molecular understanding of this process, we might be able to harness it for conditions including infectious diseases, transplantation, autoimmune disorders and cancer.”
B cells are a type of white blood cell that floats around the body, looking for foreign pathogens, such as viruses or even cells from a transplanted organ. When they encounter an invader, B cells begin pumping out antibodies, which are proteins that fit onto the pathogen, blocking it from entering the body’s cells and tagging it for destruction.
Over the course of several weeks, B cells learn and evolve to make antibodies that are a better and better fit to the pathogen.
Through a series of single-cell genomic experiments that examined evolving B cells, Singh and his team – which included scientists at Westlake University in Hangzhou, China – were able to find patterns of gene activity that distinguished the B cells that were being better evolved for the immune response versus those that were not. The better B cells changed the activities of genes that control mitochondrial activity and cell division, suggesting that this molecular coordination enabled B cells with a higher affinity for the pathogen to proliferate more rapidly, outcompeting B cells with lower affinity. By turning down or turning up mitochondrial activity, the research team showed that they could control how rapidly B cells evolve.
B cell evolution is the process the body goes through after most types of vaccination. Within a few weeks of being immunized, people with a strong response to the vaccine will have specialized B cells that have evolved to produce antibodies that are a better fit for the pathogen being vaccinated against.
It is also why people who receive organ transplants must take immunosuppressive drugs – otherwise B cells will begin producing antibodies that target cells in the transplanted organ for destruction.
“Now that we have new insights into how B cell evolution works, we can explore how to rationally accelerate this process, leading to more effective vaccines,” said Singh, who began the work at Cincinnati Children’s Hospital Medical Center. “Alternatively, if we need to stop the immune system from attacking a transplanted organ, we would want to diminish the response. Knowing the underlying mechanisms opens up a world of possibilities.”
In addition to the UPMC ITTC, this research was supported by the National Natural Science Foundation of China, the National Key R&D Program of China, the Education Foundation of Westlake University and the National Institutes of Health.