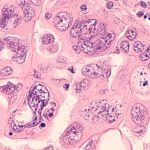
A new study by University of Pittsburgh researchers sheds light on how the oxygen-binding molecule heme, which is released from the fragile red blood cells of sickle cell disease (SCD) patients, could cause chronic kidney disease, a serious complication that affects more than half of patients with SCD.
Published in a recent issue of the journal Blood, the study also identified a protein that may protect against kidney disease in mice and patients, suggesting it has potential as both a biomarker to identify at-risk patients and as a novel target for new therapeutics.
“Patients with sickle cell disease have a life expectancy of just 50 to 55 years, almost two decades less than the general population,” said senior author Dr. Samit Ghosh, assistant professor in the Department of Medicine and member of the Vascular Medicine Institute at Pitt. “Chronic kidney disease is a major contributor to mortality in these patients, so there is a huge need to better understand the mechanisms driving disease and develop new therapies.”
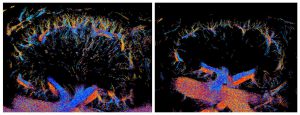
Super-resolution ultrasound imaging showing how repeated exposure to heme leads to extensive loss of small blood vessels (right) in the kidneys of mice with sickle cell disease compared to those that didn’t receive heme (left). Credit: Qiyang Chen & Kang Kim
SCD patients produce a flawed version of hemoglobin, the protein in red blood cells that carries oxygen around the body, that is shaped like a crescent, or sickle, instead of a round disc. These stiff, sticky cells block blood flow by clumping together and are prone to breakage, or hemolysis, which results in heme leaking into the bloodstream.
“When heme is inside red blood cells, it’s essential for carrying oxygen to different tissues around the body,” explained Ghosh. “But when heme is released out of the cell via hemolysis, it’s toxic to organs and tissues.”
To learn more about how hemolysis contributes to kidney disease, Ghosh and his team used a mouse model of SCD that expresses human sickle hemoglobin. They infused the animals with heme repeatedly over time to mimic ongoing hemolysis that occurs in SCD patients.
Dr. Kang Kim, professor in the Department of Bioengineering and Department of Medicine at Pitt and member of Pitt’s Vascular Medicine Institute and the UPMC Heart & Vascular Institute, and first author Dr. Qiyang Chen, postdoctoral associate in Dr. Kim’s Lab, then used a novel super-resolution ultrasound imaging to non-invasively measure the extent of vascular damage in the animals.
They found that repeated exposure to heme led to damage of the renovascular endothelium, the layer of cells lining the kidney’s blood vessels, and renal failure. The findings suggest that ongoing hemolysis, experienced by SCD patients, drives chronic kidney disease.
Delving deeper, the researchers found that a protein called EPCR was cleaved from the surface of endothelial cells in response to heme. As a result, these mice had more EPCR in their bloodstream.
Blood levels of EPCR were also elevated in SCD patients with chronic kidney disease, mirroring the results in mice.
“Our findings suggest that blood levels of EPCR could act as a potential biomarker of chronic kidney disease in patients with SCD,” said Ghosh. “If we can predict who is at greater risk for kidney disease, we could treat these patients earlier to help prevent disease progression.”
It’s not clear how heme causes EPCR to detach from the cell surface, but Ghosh has several hypotheses that he plans to investigate in future research.
“Different people seem to have different degrees of EPCR stability,” said Ghosh. “Some have EPCR that is harder to cleave from the endothelium, and we hypothesize they may be more protected from kidney disease than those with EPCR that is more easily detached. We are now planning to test this.”
Using mice that overexpressed EPCR, the researchers found that the protein protected animals from heme damage.
According to Ghosh, these findings suggest that drugs or gene therapies that induce higher levels of EPCR in the endothelium could potentially be a new approach for treating or preventing chronic kidney disease and damage to other organs in patients with SCD.